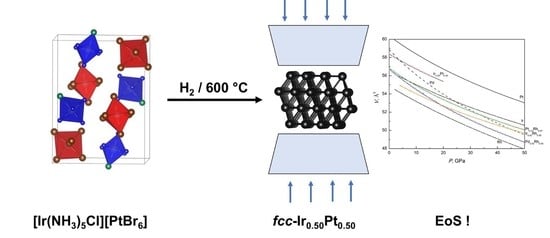
Face-Centered Cubic Refractory Alloys Prepared from Single-Source Precursors
Abstract
:1. Introduction
2. Materials and Methods
| Composition | Ir+Pt, wt %, Calculated | Ir+Pt, wt %, Obtained |
| [Ir(NH3)5Cl][PtBr6] | 39.23 | 40.3 ± 0.2 |
| [Ir(NH3)5Cl][PtCl6] | 53.75 | 53.3 ± 0.2 |
3. Results and Discussion
3.1. Crystal Structures of [Ir(NH3)5Cl][PtCl6] and [Ir(NH3)5Cl][PtBr6] Single-Source Precursors
3.2. Preparation of fcc-Structured Binary Alloys under Ambient Pressure and Their Phase Composition
3.3. High-Pressure Compressibility of fcc-Structured Binary Refractory Alloys
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yusenko, K.V.; Riva, S.; Carvalho, P.A.; Yusenko, M.V.; Arnaboldi, S.; Sukhikh, A.; Hanfland, M.; Gromilov, S.A. First hcp HEA with outstanding stability under extreme conditions and high electrocatalytic activity in methanol oxidation. Scripta Materialia 2017, 138, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Occelli, F.; Farber, D.L.; Badro, J.; Aracne, C.M.; Teter, D.M.; Hanfland, M.; Canny, B.; Couzinet, B. Experimental evidence for a high-pressure isostructural phase transition in osmium. Phys. Rev. Lett. 2004, 93, 095502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrovinsky, L.; Dubrovinskaia, N.; Prakapenka, V.B.; Abakumov, A.M. Implementation of micro-ball nanodiamond anvils for high-pressure studies above 6 Mbar. Nat. Commun. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dubrovinsky, L.; Dubrovinskaia, N.; Bykova, E.; Bykov, M.; Prakapenka, V.; Prescher, C.; Glazyrin, K.; Liermann, H.-P.; Hanfland, M.; Ekholm, M.; et al. The most incompressible metal osmium at static pressures above 750 gigapascals. Nature 2015, 525, 226–229. [Google Scholar] [CrossRef]
- Yusenko, K.V.; Khandarkhaeva, S.; Fedotenko, T.; Pakhomova, A.; Gromilov, S.A.; Dubrovinsky, L.; Dubrovinskaia, N. Equations of state of Rhodium, Iridium and their alloys up to 70 GPa. J. Alloy. Comp. 2019, 788, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Yusenko, K.V.; Bykova, E.; Bykov, M.; Gromilov, S.A.; Kurnosov, A.V.; Prescher, C.; Prakapenka, V.B.; Hanfland, M.; van Smaalen, S.; Margadonna, S.; et al. Compressibility of Ir–Os alloys under high pressure. J. Alloy. Comp. 2015, 622, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Yusenko, K.V.; Bykova, E.; Bykov, M.; Gromilov, S.A.; Kurnosov, A.V.; Prescher, C.; Prakapenka, V.B.; Crichton, W.A.; Hanfland, M.; Margadonna, S.; et al. High-pressure high-temperature stability of hcp-IrxOs1-x (x = 0.50 and 0.55) alloys. J. Alloy. Comp. 2017, 700, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Yusenko, K.V.; Bykova, E.; Bykov, M.; Riva, S.; Crichton, W.A.; Yusenko, M.V.; Sukhikh, A.S.; Arnaboldi, S.; Hanfland, M.; Dubrovinsky, L.S.; et al. Ir-Re binary alloys under extreme conditions and their electrocatalytic activity in methanol oxidation. Acta Mater. 2017, 139, 236–243. [Google Scholar] [CrossRef]
- Shubin, Y.V.; Korenev, S.V.; Yusenko, K.V.; Korda, T.M.; Venediktov, A.B. X-ray study of double complex salts [M(NH3)5Cl][M*Cl4] – precursors of polymetallic powders (M = Ir, Rh, Co; M* = Pt, Pt). Russ. Chem. Bull. 2002, 51, 41–45. [Google Scholar] [CrossRef]
- Bykov, M.; Yusenko, K.V.; Bykova, E.; Pakhomova, A.; Kraus, W.; Dubrovinskaia, N.; Dubrovinsky, L. Synthesis of arsenopyrite-type rhodium pernitride RhN₂ from a single-source azide precursor. Eur. J. Inorg. Chem. 2019, 32, 3667–3671. [Google Scholar] [CrossRef]
- Garnier, E. Structure of Bis[pentaamminechloroiridium(III)] Hexachloroplatinate(IV) Dicloride. Acta Cryst. C. 1993, C49, 578–580. [Google Scholar] [CrossRef]
- Yusenko, K.V.; Gromilov, S.A.; Baidina, I.A.; Shubin, Y.V.; Korolkov, I.V.; Drebushchak, T.N.; Basova, T.V.; Korenev, S.V. Synthesis, structure, and thermal decomposition of chloropentamminerhodium(III) hexabromoplatinate(IV). J. Struct. Chem. 2002, 43, 649–655. [Google Scholar] [CrossRef]
- Yusenko, K.V.; Gromilov, S.A.; Korenev, S.V.; Baidina, I.A.; Korolkov, I.V.; Drebushchak, T.N. Synthesis and crystal structure of [Rh(NH3)5Cl]2[PtCl6]Cl2. J. Struct. Chem. 2002, 43, 697–699. [Google Scholar] [CrossRef]
- Gromilov, S.A.; Korenev, S.V.; Baidina, I.A.; Korolkov, I.V.; Yusenko, K.V. Syntheses of [Rh(NH3)5Cl][MCl6] (M = Re, Os, Ir) and investigation of their thermolysis products. Crystal structure of [Rh(NH3)5Cl][OsCl6]. J. Struct. Chem. 2002, 43, 488–494. [Google Scholar] [CrossRef]
- Yusenko, K.V.; Shusharina, E.A.; Gromilov, S.A. X-ray diffraction study of [MI(NH3)5][MIIBr6] (MI = Rh, Ir; MII = Re, Ir) polycrystals. J. Struct. Chem. 2010, 51, 935–941. [Google Scholar] [CrossRef]
- Yusenko, K.V.; Gromilov, S.A.; Baidina, I.A.; Korolkov, I.V.; Zhivonitko, V.V.; Venediktov, A.B.; Korenev, S.V. Crystal structure of [M(NH3)5Cl]2[IrCl6]Cl2 (M = Co, Rh, Ir) binary complex salts. J. Struct. Chem. 2003, 44, 60–67. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General Features. Z. Krist 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Angel, R.J. Equations of State. In Hazen, R.M., Downs, R.T. (Eds.). High-pressure, high-temperature crystal chemistry. Rev. Mineral. Geochem. 2001, 41, 35–60. [Google Scholar] [CrossRef]
- Korenev, S.V.; Venediktov, A.B.; Shubin, Y.V.; Gromilov, S.A.; Yusenko, K.V. Synthesis and structure of binary complexes of platinum group metals – precursors of metallic materials. J. Struct. Chem. 2003, 44, 46–59. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Domonov, D.P. Properties of binary complex compounds. J Struct. Chem. 2011, 52, 412–427. [Google Scholar] [CrossRef]
- Darling, A.S.; Mech, A.M.I. Rhodium Platinum Alloys. A Critical Review of Their Constitution and Properties. Platin. Met. Rev. 1961, 5, 58–65. [Google Scholar]
- Tripathi, S.N.; Bharadwaj, S.R.; Chandrasekharaia, M.S. The Ir—Rh (Iridium—Rhodium) system. J. Phase Equilibria 1991, 12, 606–608. [Google Scholar] [CrossRef]
- Gürler, R.; Cornish, L.A.; Pratt, J.N. Computer assessment of the palladium-rhodium system. J. Alloys Comp. 1993, 191, 165–168. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Aoyagi, T.; Abe, T. An investigation of phase separation in the Ir–Pt binary system. J. Alloys Comp. 2009, 484, 327–334. [Google Scholar] [CrossRef]
- Venediktov, A.B.; Korenev, S.V.; Shubin, Y.V.; Kuznetsov, N.A.; Yusenko, K.V. Synthesis and properties of double complexes [M(NH3)5Cl][PdBr4] (M = Co, Rh, Ir). Russ. J. Inorg. Chem. 2003, 48, 379–384. [Google Scholar]
- Zen E-an. Validity of “Vegard’s Law”. Amer. Min. 1956, 41, 523–524. [Google Scholar]
- Denton, A.R.; Ashcroft, N.W. Vegard’s Law. Phys. Rev. A 1991, 43, 3161. [Google Scholar] [CrossRef]
- Yusenko, K.V. Phase diagram of the iridium—rhenium metallic system. Platin. Met. Rev. 2013, 57, 57–65. [Google Scholar] [CrossRef]
- Yusenko, K.V. Phase diagram of the rhenium—rhodium system: State of the art. Platin. Met. Rev. 2011, 54, 188–194. [Google Scholar] [CrossRef]
- Varotsos, P.; Alexopoulos, K. Series, Defects in solids. In Thermodynamics of point defects and their relation with bulk properties; Amelinckx, S., Gevers, R., Nihoul, J., Eds.; North-Holland Publ. Co.: Amsterdam, The Netherlands, 1986; pp. 156–158, 325–347. [Google Scholar]
- Introduction to the physics of the Earth’s interior. Available online: http://www.doganaydal.com/nesneler/kutuphanekitaplar/INTRODUCTION_TO_THE_PHYSICS_OF_THE_EARTHS_INTERIOR.PDF (accessed on 9 January 2020).
- Yingwei, F.; Ricolleau, A.; Frank, M.; Mibe, K.; Shen, G.; Prakapenka, V. Toward an internally consistent pressure scale. PNAS 2007, 104, 9182–9186. [Google Scholar]
- Mao, H.K.; Bell, P.M.; Shaner, J.W.; Steinberg, D.J. Specific volume measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R1 fluorescence pressure gauge from 0.06 to 1 Mbar. J. Appl. Phys. 1978, 49, 3276–3283. [Google Scholar] [CrossRef]
- Domonov, D.P.; Pechenyuk, S.I.; Belyaevskii, A.T.; Yusenko, K.V. Formation of Nanostructured Carbon from [Ni(NH3)6]3[Fe(CN)6]2. Nanomaterials 2020, 10, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domonov, D.P.; Pechenyuk, S.I.; Semushina, Y.P.; Yusenko, K.V. Solid-state transformations in inner coordination sphere of [Co(NH3)6][Fe(C2O4)3]∙3H2O as a route to access catalytically active Co-Fe materials. Materials 2019, 12, 221. [Google Scholar] [CrossRef] [Green Version]





| Single-Source Precursor Ref. | Preparatory Conditions | Phase Composition | a, Å* | V/Z, Å3·atom−1 | Δa, Å |
|---|---|---|---|---|---|
| [Ir(NH3)5Cl][PtCl6] | He flow, 400 °C | two fcc phases | aI = 3.919(6) aII = 3.847(6) | — | — |
| H2 flow, 700 °C | fcc–Ir0.50Pt0.50 sample A | 3.883(2) | 14.537 | −0.08 | |
| [Ir(NH3)5Cl][PtBr6] | He flow, 600 °C | two fcc phases | — | — | — |
| H2 flow, 500 °C | fcc–Ir0.50Pt0.50 | 3.874(2) | 14.535 | −0.085 | |
| [Ir(NH3)5Cl]2[PtCl6]Cl2 [11] (only crystal structure was reported) | He flow, 460 °C | two fcc phases | aI = 3.855 aII = 3.917 | — | — |
| H2 flow, 500 °C | fcc–Ir0.67Pt0.33 | 3.870(1) | 14.490 | 0.03 | |
| [Rh(NH3)5Cl][PtCl6] | He flow, 600 °C | fcc–Rh0.72Pt0.28 fcc–Rh0.79Pt0.21 | aI = 3.837(4) aII = 3.828(4) | — | — |
| H2 flow, 550 °C | fcc–Rh0.5Pt0.5 | 3.865(4) | 14.434 | 0.002 | |
| [Rh(NH3)5Cl][PtBr6] [12] | He flow, 800 °C | fcc–Rh0.38Pt0.62 fcc–Rh0.72Pt0.28 | aI = 3.878(3) aII = 3.836(3) | — | — |
| H2 flow, 700 °C | fcc–Rh0.50Pt0.50 | 3.864(2) | 14.423 | 0.001 | |
| [Rh(NH3)5Cl]2[PtCl6]Cl2 [13] | He flow, 460 °C | fcc–Rh0.03Pt0.97 fcc–Rh0.93Pt0.07 | aI = 3.919(5) aII = 3.811(5) | — | — |
| H2 flow, 500 °C | fcc–Rh0.66Pt0.33 sample B | 3.845(5) | 14.211 | 0.001 | |
| [Rh(NH3)5Cl][IrCl6] [5,14] | Ar flow, 550 °C | fcc–Rh0.50Ir0.50 | 3.817(2) | 13.903 | −0.004 |
| H2 flow 650 °C | fcc–Rh0.50Ir0.50 | 3.825(2) | 13.991 | 0.004 | |
| [Rh(NH3)5Cl][IrBr6] [15] | He flow, 800 °C | fcc–Rh0.50Ir0.50 | 3.820(2) | 13.936 | 0.001 |
| H2 flow, 600 °C | fcc–Rh0.50Ir0.50 | 3.824(2) | 13.980 | 0.003 | |
| [Rh(NH3)5Cl]2[IrCl6]Cl2 [16] | He flow, 470 °C | fcc–Rh0.66Ir0.33 | 3.810(2) | 13.827 | −0.005 |
| H2 flow, 700 °C | fcc–Rh0.66Ir0.33 | 3.813(4) | 13.859 | −0.002 | |
| [Rh(NH3)5Cl][PdCl4]·H2O [9] | H2 flow, 400 °C | fcc–Rh0.50Pd0.50 sample C | 3.845(4) | 14.211 | −0.002 |
| Composition | [Ir(NH3)5Cl][PtCl6] Powder, RT | [Ir(NH3)5Cl][PtBr6] Single Crystal, 150 K | [Rh(NH3)5Cl][IrCl6] Powder, RT | [Rh(NH3)5Cl][IrBr6] Single Crystal, RT | [Rh(NH3)5Cl][PtBr6] Single Crystal, RT |
|---|---|---|---|---|---|
| a, Å | 11.568(3) | 11.9099(13) | 11.67(6) | 12.030(6) | 12.013(2) |
| b, Å | 8.314(2) | 8.3277(9) | 8.348(7) | 8.532(5) | 8.401(2) |
| c, Å | 16.104(3) | 15.832(2) | 15.65(3) | 16.382(6) | 15.999(3) |
| b, ° | 110.15(5) | 90.000(4) | 105.7(3) | 106.23(1) | 91.13(3) |
| V, Å3 | 1454.1 | 1570.3(3) | 1468.0 | 1614.4 | 1614.4 |
| Space group | P21/m | P21/m | P21/m | P21/m | P21/m |
| Z | 4 | 4 | 4 | 4 | 4 |
| Molecular weight | 720.62 | 987.32 | 628.45 | 895.15 | 898.01 |
| D, g/cm3 | 3.287 | 4.176 | 2.844 | 3.683 | 3.70 |
| PDF number | — | — | — | 00-057-086501-080-8875 | 01-072-8177 |
| ICSD number | — | 1971298 | — | 421153 | 98115 |
| Reference | present study | present study | [14] | [15] | [12] |
| Composition (max. P) | V0/Z, Å3·atom−1 (P = 1 bar)b | V0/Z, Å3·atom−1 According to Zen’s Rule | B0, GPa B0’ | B0, GPa According to Equation (2) | Ref. |
|---|---|---|---|---|---|
| fcc–Ir0.42Rh0.58 (up to 57 GPa) | 13.90(8) | 13.909 | 317(17) 6.0(5) | 316.9 | [5] |
| fcc–Ir0.50Pt0.50 (up to 15 GPa) | 14.597(6) | 14.625 | 321(6) 6(1) | 304.7 | Sample A |
| fcc–Pd0.50Rh0.50 (up to 45 GPa) | 14.18(2) | 14.224 | 223(4) 5.0(3) | 225.7 | Sample C |
| fcc–Pt0.33Rh0.67 (up to 47 GPa) | 14.211(3) | 14.180 | 259(1) 6.66(9) | 292.1 | Sample B |
| fcc–Ir (up to 67 GPa) | 14.14(6) | ― | 341(10) 4.7(3) | ― | [5] |
| fcc–Rh (up to 64 GPa) | 13.73(7) | ― | 301(9) 3.1(2) | ― | [5] |
| fcc–Pt (up to 100 GPa) | 15.094(2) | ― | 277(2) 4.95(2) | ― | [33] |
| fcc–Pd (up to 100 GPa) | 14.718(2) | ― | 183 5.28 | ― | [34] |
| fcc–Ir0.26Os0.05Pt0.31Rh0.23Ru0.15 (up to 49 GPa) | 14.16(9) | 14.262 | 300(22) 6(1) | ― | [5] |
| hcp–Ir0.24Os0.21Re0.16Rh0.18Ru0.20 (up to 45 GPa) | 13.979(2) | 13.882 | 317(2) 4.9(1) | ― | [1] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusenko, K.V.; Khandarkhaeva, S.; Bykov, M.; Fedotenko, T.; Hanfland, M.; Sukhikh, A.; Gromilov, S.A.; Dubrovinsky, L.S. Face-Centered Cubic Refractory Alloys Prepared from Single-Source Precursors. Materials 2020, 13, 1418. https://doi.org/10.3390/ma13061418
Yusenko KV, Khandarkhaeva S, Bykov M, Fedotenko T, Hanfland M, Sukhikh A, Gromilov SA, Dubrovinsky LS. Face-Centered Cubic Refractory Alloys Prepared from Single-Source Precursors. Materials. 2020; 13(6):1418. https://doi.org/10.3390/ma13061418
Chicago/Turabian StyleYusenko, Kirill V., Saiana Khandarkhaeva, Maxim Bykov, Tymofey Fedotenko, Michael Hanfland, Alexander Sukhikh, Sergey A. Gromilov, and Leonid S. Dubrovinsky. 2020. "Face-Centered Cubic Refractory Alloys Prepared from Single-Source Precursors" Materials 13, no. 6: 1418. https://doi.org/10.3390/ma13061418
APA StyleYusenko, K. V., Khandarkhaeva, S., Bykov, M., Fedotenko, T., Hanfland, M., Sukhikh, A., Gromilov, S. A., & Dubrovinsky, L. S. (2020). Face-Centered Cubic Refractory Alloys Prepared from Single-Source Precursors. Materials, 13(6), 1418. https://doi.org/10.3390/ma13061418