Melting and Recrystallization of Copper Nanoparticles Prepared by Microwave-Assisted Reduction in the Presence of Triethylenetetramine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cu-NPs
2.2. Characterization
3. Results and Discussion
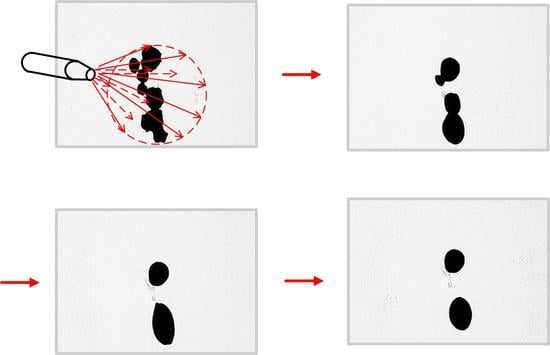
3.1. Microwave Irradiation
3.2. SEM and TEM
3.3. Wide Angled X-ray Diffraction (WXRD)
3.4. Differential Scanning Calorimeter (DSC)
3.5. Thermogravimetric Analysis (TGA)
3.6. Optical Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, Z.H.; Yang, B.; Qi, Y.; Cumings, J. Synthesis of low-melting-point metallic nanoparticles with an ultrasonic nanoemulsion method. Ultrasonics 2011, 51, 485–488. [Google Scholar] [CrossRef]
- Zhang, M.; Efremov, M.; Schiettekatte, F.; Olson, E.A.; Kwan, A.T.; Lai, S.L.; Wisleder, T.; Greene, J.E.; Allen, L.H. Size-dependent melting point depression of nanostructures: Nanocalorimetric measurements. Phys. Rev. B 2000, 620, 10548–10557. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wang, W.; Yue, Q. Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.R.; Sharma, A.K. Microwave–material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos. Part A 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Figlarz, M.; Fievet, F.; Lagier, J.P. Process for the Reduction of Metallic Compounds by Polyols, and Metallic Powders Obtained by this process. U.S. Patent No. 4,539,041, 3 September 1985. [Google Scholar]
- Chow, G.M.; Schoen, P.E.; Kurihara, L.K. Nanostructured metallic powders and films via an alcoholic solvent process. U.S. Patent No. 5,759,230, 2 June 1998. [Google Scholar]
- Kurihara, L.K.; Chow, G.M.; Schoen, P.E. Nanocrystalline metallic powders and films produced by the polyol method. NanaShuchued Mater. 1995, 5, 607–613. [Google Scholar] [CrossRef]
- Zhu, H.T.; Lin, Y.S.; Yin, Y.S. A novel one−step chemical method for preparation of copper nanofluids. J. Colloid Interface Sci. 2004, 277, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.M.D.; Le, T.T.T.; Blanc, E.F.; Dang, M.C. Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Huang, H.H.; Yan, F.Q.; Kek, Y.M.; Chew, C.H.; Xu, G.Q.; Ji, W.; Oh, P.S.; Tang, S.H. Synthesis, characterization, and nonlinear optical properties of copper nanoparticles. Langmuir 1997, 13, 172–175. [Google Scholar] [CrossRef]
- Aslam, M.; Gopakumar, G.; Shoba, T.L.; Mulla, I.S.; Vijayamohanan, K.; Kulkarni, S.K.; Urban, J.; Vogel, W. Formation of Cu and Cu2O nanoparticles by variation of the surface ligand: Preparation, structure, and insulating−to−metallic transition. J. Colloid Interface Sci. 2002, 255, 79–90. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Dmitruk, I.M. Size−dependent melting of spherical copper nanoparticles embedded in a silica matrix. Phys. Rev. B 2007, 75, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Blosi, M.; Albonetti, S.; Dondi, M.; Martelli, C.; Baldi, G. Microwave−assisted polyol synthesis of Cu nanoparticles. J. Nanopart. Res. 2011, 13, 127–138. [Google Scholar] [CrossRef]
- Visurraga, J.D.; Plessing, C.V.; Daza, C.; Pozo, C.; Becerra, A.; Garcia, A. Study on antibacterial alginate−stabilized copper nanoparticles by FT-IR and 2D-IR correlation spectroscopy. Int. J. Nanomed. 2012, 7, 3597–3612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzemann, C.; Lisiecki, I.; Urban, J.; Pileni, M.P. Anisotropic copper nanocrystals synthesized in a supersaturated medium: Nanocrystal growth. Langmuir 2004, 20, 11772–11777. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Kosaka, Y.; Myoujin, Y.; Narushima, T.; Yonezawa, T.; Arakawa, R. Microwave−assisted polyol synthesis of copper nanocrystals without using additional protective agents. Chem. Commun. 2011, 47, 7740–7742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreder, K.J.; Manthiram, A. Metal nanofoams via a facile microwave−assisted solvothermal process. Chem. Commun. 2016, 53, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Anzlvar, A.; Orel, Z.C.; Zigon, M. Copper(I) oxide and metallic copper particles formed in 1,2−propane diol. J. Eur. Ceram. Soc. 2007, 27, 987–991. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Vesaghi, M.A.; Shafiekhani, A. Study of surface plasmon resonance of Cu@Cu2O core–shell nanoparticles by Mie theory. J. Phys. D Appl. Phys. 2009, 42, 1–6. [Google Scholar] [CrossRef]
- Cottancin, E.; Celep, G.; Leré, J.; Pellarin, M.; Huntzinger, J.R.; Vialle, J.L.; Broyer, M. Optical properties of noble metal clusters as a function of the size: Comparison between experiments and a semi−quantal theory. Theor. Chem. Acc. 2006, 116, 514–523. [Google Scholar] [CrossRef]
- Wei, W.; Lu, Y.; Chen, W.; Chen, S. One−pot synthesis, photoluminescence, and electrocatalytic properties of subnanometer−sized copper clusters. J. Am. Chem. Soc. 2011, 133, 2060–2063. [Google Scholar] [CrossRef]
- Vidal, N.V.; Blanco, M.C.; López-Quintela, M.A.; Rivas, J.; Serra, C. Electrochemical synthesis of very stable photoluminescent copper clusters. J. Phys. Chem. C 2010, 114, 15924–15930. [Google Scholar] [CrossRef]
- Vazquez, C.; Banobre–Lopez, M.; Mitra, A.; Lopez–Quintela, M.A.; Rivas, J. Synthesis of small atomic copper clusters in microemulsions. Langmuir 2009, 25, 8208–8216. [Google Scholar] [CrossRef]
- Haram, S.K.; Mahadeshwar, A.R.; Dixit, S.G. Synthesis and characterization of copper sulfide nanoparticles in Triton-X 100 water-in-oil microemulsions. J. Phys. Chem. 1996, 100, 5868–5873. [Google Scholar] [CrossRef]
- Lisiecki, I.; Pileni, M.P. Synthesis of Copper Metallic Clusters Using Reverse Micelles as Microreactors. J. Am. Chem. Soc. 1993, 115, 3887–3896. [Google Scholar] [CrossRef]
- Pileni, M.P.; Lisiecki, I. Nanometer Metallic Copper Particle Synthesis in Reverse Micelles. Colloids Surf. Physicochem. Eng. Asp. 1993, 80, 63–68. [Google Scholar] [CrossRef]
- Balogh, L.; Tomalia, D.A. Poly(amidoamine) dendrimer-templated nanocomposites. 1. Synthesis of zerovalent copper nanoclusters. J. Am. Chem. Soc. 1998, 120, 7355–7356. [Google Scholar] [CrossRef]
- Ohde, H.; Hunt, F.; Wai, C.M. Synthesis of silver and copper nanoparticles in a water-in-supercritical-carbon dioxide microemulsion. Chem. Mater. 2001, 13, 4130–4135. [Google Scholar] [CrossRef]
- Cheon, J.M.; Lee, J.H.; Song, Y.; Kim, J. Snthesis of Ag nanoparticles using an electrolysis method and application to inkjet printing. Colloids Surf. Physicochem. Eng. Asp. 2011, 389, 175–179. [Google Scholar] [CrossRef]
- Kumar, R.V.; Mastai, Y.; Diamant, Y.; Gedanken, A. Sonochemical synthesis of amorphous Cu and nanocrystalline Cu2O embedded in a polyaniline matrix. J. Mater. Chem. 2001, 11, 1209–1213. [Google Scholar] [CrossRef]
- Tseng, P.H.; Wang, Y.Z.; Hsieh, T.H.; Ho, K.S.; Tsai, C.H.; Chen, K.T. Preparation of low size copper nanoparticles by microwave irradiation in the presence of triethylene tetramine. Nanotechnology 2018, 29, 085603. [Google Scholar] [CrossRef]
- Schmid, G.; Corain, B.E. Nanoparticulated Gold: Syntheses, Structures, Electronics, and Reactivities. Eur. J. Inorg. Chem. 2003, 2003, 3081–3098. [Google Scholar] [CrossRef]
- Castro, T.; Reifenberger, R.; Choi, E.; Andres, R.P. Size-dependent melting temperature of individual nanometer-sized metallic clusters. Phys. Rev. B 1990, 13, 8548–8556. [Google Scholar] [CrossRef]
- Cisneros, R.; Ramírez, C.; Wang, C.-M. Ellipsometry and ab initio approaches to the refractive index of porous silicon. J. Phys. Condens. Matter 2007, 19, 395015. [Google Scholar] [CrossRef]
- Zola, A.S.; Ribeiro, R.U.; Bueno, J.M.C.; Zanchet, D.; Arroyo, P.A. Cobalt nanoparticles prepared by three different methods. J. Exp. Nanosci. 2014, 9, 398–405. [Google Scholar] [CrossRef]
- Kelton, K.F.; Lee, G.W.; Gangopadhyay, A.K.; Hyers, R.W.; Rathz, T.J.; Rogers, J.R.; Robinson, M.B.; Robinson, D.S. First X-ray scattering studies on electrostatically levitated metallic fluids: Demonstrated influence of local icosahedral order on the nucleation barrier. Phys. Rev. Lett. 2003, 90, 19550. [Google Scholar] [CrossRef]
- Mei, Q.S.; Lu, K. Melting and superheating of crystalline solids: From bulk to nanocrystals. Prog. Mater. Sci. 2007, 52, 1175–1262. [Google Scholar] [CrossRef]
- Dash, J.G. History of the search for continuous melting. Rev. Mod. Phys. 1999, 71, 1737–1743. [Google Scholar] [CrossRef]
- Shidpour, R.; Delavari, H.H.; Vossoughi, M. Analytical model based on cohesive energy to indicate the edge and corner effects on melting temperature of metallic nanoparticles. Chem. Phys. 2010, 378, 14–18. [Google Scholar] [CrossRef]
- Qi, W.H.; Huang, B.Y.; Wang, M.P.; Li, Z.; Yu, Z.M. Generalized bondenergy model for cohesive energy of small metallic particles. Phys. Lett. A 2007, 370, 494–498. [Google Scholar] [CrossRef]
- Shandiz, M.A.; Safaei, A.; Sanjabi, S.; Barber, Z.H. Modeling the cohesive energy and melting point of nanoparticles by their average coordination number. Solid State Commun. 2008, 145, 432–437. [Google Scholar] [CrossRef]
- Chakravarty, C.; Debenedetti, P.G.; Stillinger, F.H. Lindemann measures for the solid-liquid phase transition. J. Chem. Phys. 2007, 126, 204508. [Google Scholar] [CrossRef]
- Pauling, L. So-called icosahedral and decagonal quasicrystals are twins of an 820-atom cubic crystal. Phys. Rev. Lett. 1987, 58, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Fraundorf, P.; Bishop, C. Efficient Lattice-Image Detection of Icosahedral Twins. Microsc. Microanal. 2013, 19, 1804–1805. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.L.; Zeng, Q.; Zhang, T.T.; Yang, M.L.; Jackson, K.A. Icosahedral to double-icosahedral shape transition of copper clusters. J. Chem. Phys. 2012, 136, 104501. [Google Scholar] [CrossRef] [PubMed]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jheng, L.-C.; Wang, Y.-Z.; Huang, W.-Y.; Ho, K.-S.; Tsai, C.-H.; Huang, C.-T.; Tsai, H.-S. Melting and Recrystallization of Copper Nanoparticles Prepared by Microwave-Assisted Reduction in the Presence of Triethylenetetramine. Materials 2020, 13, 1507. https://doi.org/10.3390/ma13071507
Jheng L-C, Wang Y-Z, Huang W-Y, Ho K-S, Tsai C-H, Huang C-T, Tsai H-S. Melting and Recrystallization of Copper Nanoparticles Prepared by Microwave-Assisted Reduction in the Presence of Triethylenetetramine. Materials. 2020; 13(7):1507. https://doi.org/10.3390/ma13071507
Chicago/Turabian StyleJheng, Li-Cheng, Yen-Zen Wang, Wen-Yao Huang, Ko-Shan Ho, Cheng-Hsien Tsai, Ching-Tang Huang, and Huang-Shian Tsai. 2020. "Melting and Recrystallization of Copper Nanoparticles Prepared by Microwave-Assisted Reduction in the Presence of Triethylenetetramine" Materials 13, no. 7: 1507. https://doi.org/10.3390/ma13071507
APA StyleJheng, L. -C., Wang, Y. -Z., Huang, W. -Y., Ho, K. -S., Tsai, C. -H., Huang, C. -T., & Tsai, H. -S. (2020). Melting and Recrystallization of Copper Nanoparticles Prepared by Microwave-Assisted Reduction in the Presence of Triethylenetetramine. Materials, 13(7), 1507. https://doi.org/10.3390/ma13071507