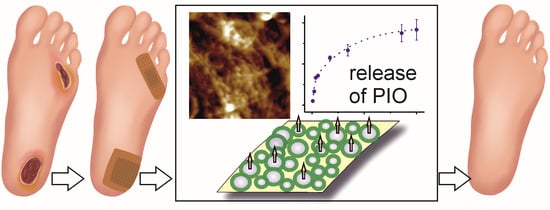
Pioglitazone-Loaded Nanostructured Hybrid Material for Skin Ulcer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PIO Solubilization-Turbidimetric Measurements
2.3. Synthesis of Hydrogel Nanoparticles Containing Surfactant Micelles with Solubilized PIO (NP-PIO)
2.4. Release Profiles Entrapment Efficiency and Loading Capacity Determination
2.5. Deposition of NP-PIO/Ch Particles on Modified Bacterial Nanocellulose (mBNC)
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. AFM Measurements
2.8. Fourier-Transform Infrared Spectroscopy (FTIR)
2.9. X-ray Diffraction (XRD) Measurements
2.10. Differential Scanning Calorimetry (DSC) Measurements
2.11. Fibroblasts Proliferation Studies
2.11.1. Cell Culture
2.11.2. Proliferation
2.11.3. Scratch Assay
3. Results and Discussion
3.1. The Influence of the Matrix Composition on Encapsulation and Release of PIO
3.2. Optimization of the PIO Loading
3.3. Studies of the Interaction of PIO with Polymer Matrix of the Nanoparticles
3.4. Deposition of NP-PIO_B/Ch(15) Nanoparticles on mBNC
3.5. Biological Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, H.; Li, C.; Jiang, Y.; Wang, B.; Wang, F.; Mao, Z.; Xu, H.; Wang, L.; Sui, X. Facile preparation of polysaccharide-based sponges and their potential application in wound dressing. J. Mater. Chem. B 2018, 6, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Wahli, W. Peroxisome Proliferator-Activated Receptors (PPARS): From metabolic control to epidermal wound healing. Swiss Med. Wkly. 2002, 132, 83–91. [Google Scholar] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Pajdo, R.; Kwiecień, S.; Konturek, S.; Targosz, A.; Burnat, G.; Cieszkowski, J.; Pawlik, W.W.; Hahn, E.G. Agonist of peroxisome proliferator-activated receptor gamma (PPAR-γ): A new compound with potent gastroprotective and ulcer healing properties. Inflammopharmacology 2005, 13, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Sen, T.; Palit, G. Involvement of glucocorticoid receptor and peroxisome proliferator activated receptor-γ in pioglitazone mediated chronic gastric ulcer healing in rats. Eur. J. Pharmacol. 2009, 609, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Moezi, L.; Janahmadi, Z.; Amirghofran, Z.; Nekooeian, A.A.; Dehpour, A.R. The increased gastroprotective effect of pioglitazone in cholestatic rats: Role of nitric oxide and tumour necrosis factor alpha. Int. J. Exp. Pathol. 2014, 95, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Burnat, G.; Szlachcic, A.; Koziel, J.; Kwiecien, S.; Konturek, S.J.; Harsch, I.A.; Clinic, T.; Agricola, G. Gastric Ulcer Healing and Stress-Lesion Preventive Properties of pioglitazone are attenuated in diabetic rats. J. Physiol. Pharmacol. 2010, 61, 429–436. [Google Scholar]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: The ‘future’ in dermatology therapeutics? Arch. Dermatol. Res. 2015, 307, 767–780. [Google Scholar] [CrossRef]
- Sakai, S.; Sato, K.; Tabata, Y.; Kishi, K. Local release of pioglitazone (a peroxisome proliferator- activated receptor c agonist) accelerates proliferation and remodeling phases of wound healing. Wound Rapair Regen. 2016, 24, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Mirza, R.E.; Fang, M.M.; Novak, M.L.; Urao, N.; Sui, A.; Ennis, W.J.; Koh, T.J. Macrophage PPARγ and impaired wound healing in type 2 diabetes. J. Pathol. 2015, 236, 433–444. [Google Scholar] [CrossRef]
- Zhang, J.U.N.; Huang, X.; Wang, L. Pioglitazone inhibits the expression of matrix metalloproteinase-9, a protein involved in diabetes-associated wound healing. Mol. Med. Rep. 2014, 10, 1084–1088. [Google Scholar] [CrossRef]
- Natarajan, J.; Sanapalli, B.K.R.; Bano, M.; Singh, S.K.; Gulati, M.; Karri, V.V.S.R. Nanostructured lipid carriers of pioglitazone loaded collagen/chitosan composite scaffold for diabetic wound healing. Adv. Wound Care 2019, 8, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Pitta, M.G.R.; Pitta, I.R.; Koh, T.J.; Abdalla, D.S.P. New Peroxisome Proliferator-Activated Receptor Agonist (GQ-11) Improves Wound Healing in Diabetic Mice. Adv. Wound Care 2019, 8, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; El-batanony, R.; Salama, A. Pioglitazone-loaded three-dimensional composite polymeric scaffolds: A proof of concept study in wounded diabetic rats. Int. J. Pharm. 2019, 570, 118667. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.M.L.; Júnior, A.R.C.; de Macedo, G.H.R.V.; Chagas, V.L.; Silva, L.D.S.; Cutrim, B.d.S.; Santos, D.M.; Soares, B.L.L.; Zagmignan, A.; de Miranda, R.d.C.M.; et al. Polysaccharide-based formulations for healing of skin-related wound infections: Lessons from animal models and clinical trials. Biomolecules 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowska-Łańcucka, J.; Karewicz, A.; Wolski, K.Z.S. Surface Functionalization of Nanocellulose-Based Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2019; pp. 705–733. [Google Scholar]
- Gianino, E.; Miller, C.; Gilmore, J. Smart wound dressings for diabetic chronic wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Pascoal, C.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef]
- Taylor, P.; Jing, W. Laser Patterning of Bacterial Cellulose Hydrogel and its Modification With Gelatin and Hydroxyapatite for Bone Tissue Engineering Laser Patterning of Bacterial Cellulose Hydrogel and its Modification With Gelatin and Hydroxyapatite for Bone Tissue Engineering. Soft Mater. 2013, 11, 37–41. [Google Scholar]
- Uekusa, T.; Sugano, K. Journal of Pharmaceutical and Biomedical Analysis Precipitation behavior of pioglitazone on the particle surface of hydrochloride salt in biorelevant media. J. Pharm. Biomed. Anal. 2018, 161, 45–50. [Google Scholar] [CrossRef]
- Guzdek, K.; Lewandowska-Łańcucka, J.; Zapotoczny, S.; Nowakowska, M. Novel bionanocellulose based membrane protected with covalently bounded thin silicone layer as promising wound dressing material. Appl. Surf. Sci. 2018, 459, 80–85. [Google Scholar] [CrossRef]
- Rojewska, A.; Karewicz, A.; Baster, M.; Zając, M.; Wolski, K.; Kępczyński, M.; Zapotoczny, S.; Szczubiałka, K.; Nowakowska, M. Dexamethasone-containing bioactive dressing for possible application in post-operative keloid therapy. Cellulose 2019, 26, 1895–1908. [Google Scholar] [CrossRef] [Green Version]
- Rojewska, A.; Karewicz, A.; Boczkaja, K.; Wolski, K.; Kępczyński, M.; Zapotoczny, S.; Nowakowska, M. Modified bionanocellulose for bioactive wound-healing dressing. Eur. Polym. J. 2017, 96, 200–209. [Google Scholar] [CrossRef]
- Karewicz, A.; Zasada, K.; Szczubiałka, K.; Zapotoczny, S.; Lach, R.; Nowakowska, M. “Smart” alginate-hydroxypropylcellulose microbeads for controlled release of heparin. Int. J. Pharm. 2010, 385, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tapia, C.; Molina, S.; Diaz, A.; Abugoch, L.; Diaz-Dosque, M.; Valenzuela, F.; Yazdani-Pedram, M. The effect of chitosan as internal or external coating on the 5-ASA release from calcium alginate microparticles. AAPS PharmSciTech 2010, 11, 1294–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia, C.; Montezuma, V.; Yazdani-Pedram, M. Microencapsulation by spray coagulation of diltiazem HCL in calcium alginate-coated chitosan. AAPS PharmSciTech 2008, 9, 1198–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Burgess, D.J. Accelerated in-vitro release testing methods for extended-release parenteral dosage forms. J. Pharm. Pharmacol. 2012, 64, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Fontes, G.C.; Calado, V.M.A.; Rossi, A.M.; Rocha-Leão, M.H.M. Da Characterization of antibiotic-loaded alginate-osa starch microbeads produced by ionotropic pregelation. Biomed Res. Int. 2013, 2013, 472626. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.Q.; Lei, Y.S.; Song, L.M.; Yao, J.; Zhang, X.B.; Wang, X.L. Impact of amorphous and semicrystalline polymers on the dissolution and crystallization inhibition of pioglitazone solid dispersions. Powder Technol. 2013, 247, 211–221. [Google Scholar] [CrossRef]
- Jahnavi, N.; Dixit, A.; Mehta, A.K.; Mohan Varma, M. Formulation, evaluation and characterization of solid dispersions of pioglitazone hydrochloride. Indian J. Pharm. Educ. Res. 2013, 47, 113–122. [Google Scholar]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclet. Quim. 2004, 29, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm.-Drug Res. 2010, 67, 217–223. [Google Scholar]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zafiriou, S.; Stanners, S.R.; Saad, S.; Polhill, T.S.; Poronnik, P.; Pollock, C.A. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J. Am. Soc. Nephrol. 2005, 16, 638–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiński, K.; Stalińska, K.; Niziołek, A.; Wróbel, M.; Nowakowska, M.; Kaczor-Kamińska, M. Cell proliferation induced by modified cationic dextran. Bio-Algorithms Med-Syst. 2018, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]











| Sample | ALG:HPC (w/w) | Surfactant Used | Chitosan in Crosslin King Medium | C PIO [mg/mL] | dav * [µm] | LE [%] | EE [%] | Time of 50% Release [h] |
|---|---|---|---|---|---|---|---|---|
| NP-PIO_A | 3:1 | Pluronic® P103 | NO | 0.10 | 1.10 | 0.50 ± 0.03 | 43.20 ± 1.63 | 25 |
| NP-PIO_A/Ch | 3:1 | Pluronic® P103 | YES | 0.10 | 0.95 | 1.04 ± 0.02 | 72.85 ± 1.51 | 47 |
| NP-PIO_B | 4:1 | Pluronic® P103 | NO | 0.10 | 1.40 | 0.80 ± 0.03 | 56.82 ± 2.65 | 24 |
| NP-PIO_B/Ch | 4:1 | Pluronic® P103 | YES | 0.10 | 0.20 | 1.10 ± 0.01 | 77.36 ± 1.02 | 45 |
| NP-PIO_B/Ch(15) | 4:1 | Pluronic® P103 | YES | 0.15 | 0.15 | 1.35 ± 0.04 | 83.91 ± 2.68 | 25 |
| NP-PIO_B/Ch(15T) | 4:1 | Tween® 85 | YES | 0.15 | 0.70 | 1.32 ± 0.06 | 86.34 ± 2.06 | 24 |
| System | NP-PIO_B(15) | mBNC-NP-PIO_B(15) |
|---|---|---|
| Higuchi: | ||
| a | 11.56 | 15.15 |
| R2 | 0.679 | 0.865 |
| Peppas: | ||
| a | 5.56 | 10.14 |
| k | 1.04 | 0.719 |
| R2 | 0.939 | 0.917 |
| Weibull: | ||
| a | 105.92 | 91.23 |
| b | 0.00 | 0.00 |
| k | 0.05 | 0.08 |
| d | 0.88 | 0.76 |
| R2 | 0.961 | 0.898 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojewska, A.; Karewicz, A.; Karnas, K.; Wolski, K.; Zając, M.; Kamiński, K.; Szczubiałka, K.; Zapotoczny, S.; Nowakowska, M. Pioglitazone-Loaded Nanostructured Hybrid Material for Skin Ulcer Treatment. Materials 2020, 13, 2050. https://doi.org/10.3390/ma13092050
Rojewska A, Karewicz A, Karnas K, Wolski K, Zając M, Kamiński K, Szczubiałka K, Zapotoczny S, Nowakowska M. Pioglitazone-Loaded Nanostructured Hybrid Material for Skin Ulcer Treatment. Materials. 2020; 13(9):2050. https://doi.org/10.3390/ma13092050
Chicago/Turabian StyleRojewska, Agnieszka, Anna Karewicz, Karolina Karnas, Karol Wolski, Mateusz Zając, Kamil Kamiński, Krzysztof Szczubiałka, Szczepan Zapotoczny, and Maria Nowakowska. 2020. "Pioglitazone-Loaded Nanostructured Hybrid Material for Skin Ulcer Treatment" Materials 13, no. 9: 2050. https://doi.org/10.3390/ma13092050
APA StyleRojewska, A., Karewicz, A., Karnas, K., Wolski, K., Zając, M., Kamiński, K., Szczubiałka, K., Zapotoczny, S., & Nowakowska, M. (2020). Pioglitazone-Loaded Nanostructured Hybrid Material for Skin Ulcer Treatment. Materials, 13(9), 2050. https://doi.org/10.3390/ma13092050