Direct Exposure of Dry Enzymes to Atmospheric Pressure Non-Equilibrium Plasmas: The Case of Tyrosinase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. DBD Reactor and Plasma Processes
2.3. Sample Preparation, Manipulation and Enzyme Functionality Assay
2.4. Topographical and Surface Chemical Characterization of Enzyme Deposits
3. Results and Discussion
- Pure He. Helium is used in large amount, as main gas, in many AP plasma processes [27,35,36,39,51,52,53,54,55,56,57]. DBDs fed by a pure noble gas (e.g., helium or argon) are often exploited for the plasma treatment of organic materials, i.e., to induce moderate changes in the surface roughness as well as in the chemical composition of the outermost layers of these materials [27,55,56,57]. Electrical excitation conditions used in this work (Table 1) fall within the operational window of a homogeneous DBD in He (i.e., DBD in glow regime) [27,52,53,54]. Figure S1a reports voltage and current signals registered under the selected conditions; as reported in the literature on homogeneous DBDs, the current signal is characterized by only one peak per half-cycle of the applied voltage and all positive (and negative) peaks exhibit almost the same shape, amplitude, and position in the cycle [54].
- He/1% O2 mixture. Reactive oxygen species (ROSs) are effectively produced in AP non-equilibrium plasmas even at low O2 feed concentrations. They induce extensive surface chemical modifications as well as considerable etching of carbon-based materials with formation of volatile products [27,55]. Importantly, ROSs are widely known to cause biomolecule damage [36,38,40,43]. The investigation of this feed mixture allows also evaluating possible detrimental effects induced by the presence of a severe O2 contamination in the plasma. In this regard, it is worth mentioning that when plasma processes are carried out at atmospheric pressure in open-air conditions, O2 contamination inevitably occurs due to air entrainment into the plasma region [27,36]. The O2 concentration and electrical conditions used in this work (Table 1) are optimized to obtain plasma etching of polymers with uniform etching rate over the entire sample (e.g., ~55 nm·min−1 in case of a polyethylene-like coating deposited using a He-C2H4 fed DBDs). Under the selected conditions, the DBD operates in filamentary regime [27,52,53,54], without any surface damage due to discharge localization and filamentation. Figure S1b shows that, due to microdischarges formation, the current signal is formed by several peaks per half-cycle of the applied voltage, which are not characterized by the same amplitude and position in different cycles [54].
- He/C2H4 mixtures. Atmospheric pressure plasma polymerization of ethylene leads to the deposition of a hydrocarbon polymer film (also referred to as polyethylene-like film) [50,51,58] on the dry enzyme, to enable its immobilization. The investigation of He/C2H4 fed DBDs allows, therefore, evaluating whether direct plasma interaction with Tyr during the PECVD process has a negative effect on its activity. The experimental conditions used in this work (Table 1) allow depositing a uniform and smooth polymer layer over the entire sample. As far as the coatings are concerned, by changing the ethylene concentration and the applied voltage, it is possible to vary their deposition rate from ~20 to ~40 nm·min−1, without significant changes in their chemical composition and morphology [35]. With regard to the discharge regime, it is worth mentioning that at the excitation frequency of 20 kHz: (i) the DBD operates in homogeneous regime when [C2H4] and Va are lower or equal to 0.5% and 1.1 kVrms, respectively (voltage and current signals in Figure S2a,b); (ii) the DBD operates in filamentary regime when [C2H4] and Va are equal to 1% and 1.1 kVrms, respectively. Under these latter conditions, even if the current signal is characterized by only one peak per half-cycle of the applied voltage, all positive (and negative) peaks do not have the same amplitude (Figure S2c); moreover, discharge filamentation can be clearly perceived by the naked eye.
3.1. Exposure of Tyr to DBDs Fed with Pure He and He/1% O2 Mixture
3.2. Enzyme Overcoating by a Plasma-Deposited Polyethylene-Like Film
- The ethylene concentration in the feed mixture mainly influences the deposition rate of the coating. For instance, the DR increases from ~20 to ~30 nm·min−1 by increasing [C2H4] from 0.1% to 0.5%, at fixed applied voltage of 0.85 kVrms (Table S2).
3.3. Exposure of Dry Enzymes to DBDs: Comparison between Tyr and GOx
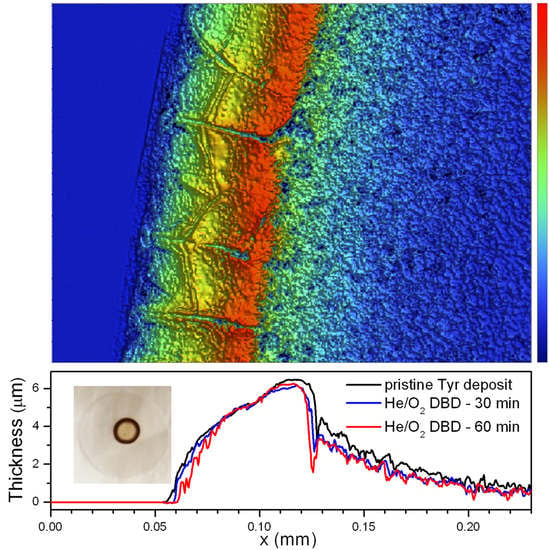
- Tyrosinase. Considerable retention of Tyr activity is observed also when very low enzyme amounts are exposed to the plasma, with weak dependence on both the enzyme exposed amount and the process duration. The He/1% O2 fed DBD appreciably affects Tyr activity when the exposed amount is below 10 µg (Figure 2). For 5 µg of dry Tyr, residual activity drops to ~65% after 30 min and remains constant for longer exposure times (Figure 3a). After 60 min of exposure to the O2-containing DBD, the 5 µg Tyr deposit shows an average thickness loss of a few hundred nm, corresponding to a residual thickness of ~90% (Figure 3a and Figure 4c). This is consistent with a remarkable etching resistance of Tyr deposits. Both residual activity and thickness decrease with exposure time (Figure 3a); however, residual thickness values remain greater than the corresponding values of residual activity. In this regard, it is found that, after plasma exposure, the major fraction of the residual enzyme exhibits unchanged functionality and substrate affinity (i.e., the slight decrease in M–M constant is found to not be statistically relevant as shown in Figure 5c), while a minor fraction is fully inactivated. The oxygen-containing DBD leads to the formation on the deposit surface of a layer enriched in inorganic compounds. This layer seems to protect tyrosinase from etching and functional damage, limiting the reduction in Tyr activity with exposure time.
- Glucose oxidase. Also for GOx it is possible to identify a threshold value of exposed amount, below which the He/1% O2 fed DBD has an appreciable impact on enzyme activity. However, in case of GOx, this threshold value is found to be 150 μg [35], i.e., fifteen times greater than that of Tyr. Moreover, a remarkable decrease in GOx residual activity is observed when the exposed amount is reduced; for instance, after 30 min plasma exposure, residual activity steeply decreases from 85% to less than 5% by decreasing the exposed amount from 100 to 10 μg. Figure 3a represents a fair comparison between the two enzymes, because although the GOx amount used in the experiments is larger (30 μg), it spreads uniformly on a larger area (8.55 mm2) so that the effective protein surface density (3.51 µg·mm−2) is very close to the surface density of Tyr in the coffee-ring (3.27 µg·mm−2). It is clear from Figure 3a that GOx residual activity more significantly decreases with exposure time, and is lower than that of Tyr. On the other hand, when GOx amount is 100 μg (i.e., a thicker enzyme deposit is exposed to the plasma), residual activity slightly decreases to ~85% after 10 min and remains constant for longer exposure times. Interestingly, in case of GOx, very good agreement is found between the values of the residual activity and residual thickness (Figure 3a). Therefore, the loss in GOx activity upon exposure to the He/1% O2 fed DBD seems to be directly correlated to the deposit thickness decrease due to etching. Moreover, it is worth mentioning that the thickness of a 30 μg GOx deposit decreases by ~3 μm after 60 min exposure to the O2-containing plasma, similarly to the polyethylene-like coating. Also for GOx, the chemical modification of the deposit surface upon plasma exposure leads to the formation of a protective layer with strong ablation resistance, which progressively reduces the etching rate with increasing the exposure time [35]. Specifically, XPS results show that the O2-containing DBD causes a decrease of both C and N surface atomic concentrations as well as considerable increase of the atomic percentages of O, K, and Na [35]. The GOx kinetic response to the O2-containing plasma is different from that shown by Tyr in similar conditions. As reported in our previous paper [35], after GOx exposure to the He/1% O2 fed DBD: (i) the decrease in Vmax (in agreement with the observed reduction in activity) corresponds to the thickness loss of the deposit, accounting for enzyme removal by plasma etching; (ii) an increase in Km is observed, suggesting that the plasma reduces the affinity for the substrate of the still functional enzyme.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Otte, K.B.; Hauer, B. Enzyme engineering in the context of novel pathways and products. Curr. Opin. Biotech. 2015, 35, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kurbanoglu, S.; Erkmen, C.; Uslu, B. Frontiers in electrochemical enzyme based biosensors for food and drug analysis. TRAC-Trends Anal. Chem. 2020, 124, 115809. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Fernández, M.; Paradisi, F. Protein immobilization technology for flow biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, J.; Liu, Z. Enhanced activity of immobilized or chemically modified enzymes. ACS Catal. 2015, 5, 4503–4513. [Google Scholar] [CrossRef]
- Geor malar, C.; Seenuvasan, M.; Kumar, K.S.; Kumar, A.; Parthiban, R. Review on surface modification of nanocarriers to overcome diffusion limitations: An enzyme immobilization aspect. Biochem. Eng. J. 2020, 158, 107574. [Google Scholar] [CrossRef]
- Mallardi, A.; Angarano, V.; Magliulo, M.; Torsi, L.; Palazzo, G. General approach to the immobilization of glycoenzyme chains inside calcium alginate beads for bioassay. Anal. Chem. 2015, 87, 11337–11344. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Jiang, T.; Wang, Y.; Wen, X.; Li, G. Constructing biopolymer-inorganic nanocomposite through a biomimetic mineralization process for enzyme immobilization. Materials 2015, 8, 6004–6017. [Google Scholar] [CrossRef] [Green Version]
- Romero-Arcos, M.; Garnica-Romo, M.G.; Martínez-Flores, H.E. Electrochemical study and characterization of an amperometric biosensor based on the immobilization of laccase in a nanostructure of TiO2 synthesized by the sol-gel method. Materials 2016, 9, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Wang, K.; Chen, Y.; Li, W.; Ye, Y.; Jin, S. Construction and characterization of a chitosan-immobilized-enzyme and β-cyclodextrin-included-ferrocene-based electrochemical biosensor for H2O2 detection. Materials 2017, 10, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowska, K.; Ciesielczyk, F.; Bachosz, K.; Zdarta, J.; Kaczorek, E.; Jesionowski, T. Laccase immobilized onto zirconia–slica hybrid doped with Cu2+ as an effective biocatalytic system for decolorization of dyes. Materials 2019, 12, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslanli, A.; Stepanov, N.; Razheva, T.; Podorozhko, E.A.; Lyagin, I.; Lozinsky, V.I.; Efremenko, E. Enzymatically functionalized composite materials based on nanocellulose and poly(vinyl alcohol) cryogel and possessing antimicrobial activity. Materials 2019, 12, 3619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apetrei, I.M.; Apetrei, C. Development of a novel biosensor based on tyrosinase/platinum nanoparticles/chitosan/graphene nanostructured layer with applicability in bioanalysis. Materials 2019, 12, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, Y.; Park, S.; Kwon, Y.H.; Ju, Y.; Yeon, K.M.; Kim, J. Tyrosinase-immobilized CNT based biosensor for highly-sensitive detection of phenolic compounds. Biosens. Bioelectron. 2019, 132, 279–285. [Google Scholar] [CrossRef]
- Elagli, A.; Belhacene, K.; Vivien, C.; Dhulster, P.; Froidevaux, R.; Supiot, P.J. Facile immobilization of enzyme by entrapment using a plasma-deposited organosilicon thin film. Mol. Catal. B 2014, 110, 77–86. [Google Scholar] [CrossRef]
- Shi, J.; Tian, Y.; Liu, H.; Yang, D.; Zhang, S.; Wu, Y.; Jiang, Z. Shielding of enzyme by a stable and protective organosilica layer on monolithic Scaffolds for Continuous Bioconversion. Ind. Eng. Chem. Res. 2017, 56, 10615–10622. [Google Scholar] [CrossRef]
- Muguruma, H.; Kase, Y. Structure and biosensor characteristics of complex between glucose oxidase and plasma-polymerized nanothin film. Biosens. Bioelectron. 2006, 22, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Belhacene, K.; Elagli, A.; Vivien, C.; Treizebré, A.; Dhulster, P.; Supiot, P.; Froidevaux, R. Investigation of the effect of plasma polymerized siloxane coating for enzyme immobilization and microfluidic device conception. Catalysts 2016, 6, 209. [Google Scholar] [CrossRef]
- Wieland, F.; Bruch, R.; Bergmann, M.; Partel, S.; Urban, G.A.; Dincer, C. Enhanced protein immobilization on polymers - A plasma surface activation study. Polymers 2020, 12, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bax, D.V.; Yin, Y.; Kondyurin, A.; Diwan, A.D.; Bhargav, D.; Weiss, A.S.; Bilek, M.M.M.; McKenzie, D.R. Plasma processing of PDMS based spinal implants for covalent protein immobilization, cell attachment and spreading. J. Mater. Sci. Mater. Med. 2018, 29, 178. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Meng, Y.; Wang, R.; Jia, B.; Li, P. Coupling and regulation of porous carriers using plasma and amination to improve the catalytic performance of glucose oxidase and catalase. Front. Bioeng. Biotechnol. 2019, 7, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coad, B.R.; Scholz, T.; Vasilev, K.; Hayball, J.D.; Short, R.D.; Griesser, H.J. Functionality of proteins bound to plasma polymer surfaces. ACS Appl. Mater. Interfaces 2012, 4, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Minier, M.J.G.; Tatoulian, M.; Chehimi, M.M.; Arefi-Khonsari, F. Ammonia plasma treated polyethylene films for adsorption or covalent immobilization of trypsin: Quantitative correlation between X-ray photoelectron spectroscopy data and enzyme activity. J. Phys. Chem. B 2011, 115, 10228–10238. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, H.; Hoshino, T.; Nowaki, K. Electronically type-sorted carbon nanotube-based electrochemical biosensors with glucose oxidase and dehydrogenase. ACS Appl. Mater. Interfaces 2015, 7, 584–592. [Google Scholar] [CrossRef]
- Fanelli, F.; Fracassi, F. Atmospheric pressure non-equilibrium plasmajet technology: Atmospheric pressure non-equilibrium plasma jet technology: Generalfeatures, specificities and applications in surface processing of materials. Surf. Coat. Technol. 2017, 322, 174–201. [Google Scholar] [CrossRef]
- Palumbo, F.; Camporeale, G.; Yang, Y.-W.; Wu, J.-S.; Sardella, E.; Dilecce, G.; Calvano, C.D.; Quintieri, L.; Caputo, L.; Baruzzi, F.; et al. Direct plasma deposition of lysozyme-embedded bio-composite thin films. Plasma Process. Polym. 2015, 12, 1302. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Hsiao, C.P.; Liu, Y.H.; Yang, C.H.; Chiang, C.Y.; Lin, T.R.; Yang, Y.W.; Wu, J.S. Enhancing adhesion and polymerization of lipase-plasma-polymerized-ethylene coatings deposited with planar dielectric-barrier-discharge-type aerosol-assisted atmospheric-pressure plasma system. Plasma Process. Polym. 2018, 15, 1700173. [Google Scholar] [CrossRef]
- Mauchauffé, R.; Bonot, S.; Moreno-Couranjou, M.; Detrembleur, C.; Boscher, N.D.; Van De Weerdt, C.; Duwez, A.-S.; Choquet, P. Fast atmospheric plasma deposition of bio-inspired catechol/quinone-rich nanolayers to immobilize NDM-1 enzymes for water treatment. Adv. Mater. Interfaces 2016, 3, 1500520. [Google Scholar] [CrossRef]
- Afshari, E.; Mazinani, S.; Ranaei-Siadat, S.-O.; Ghomi, H. Surface modification of polyvinyl alcohol/malonic acid nanofibers by gaseous dielectric barrier discharge plasma for glucose oxidase immobilization. Appl. Surf. Sci. 2016, 385, 349–355. [Google Scholar] [CrossRef]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Chen, G.; Nierstrasz, V. Surface modification of polyester fabric using plasma-dendrimer for robust immobilization of glucose oxidase enzyme. Sci. Rep. 2019, 9, 15730. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, S.; Herbert, P.A.F.; Rogalski, J.; Jaroszynska-Wolinska, J. Laccase enzyme polymerization by soft plasma jet for durable bioactive coatings. Polymers 2018, 10, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinowski, S.; Wardak, C.; Jaroszynska-Wolinska, J.; Herbert, P.A.F.; Panek, R. Cold plasma as an innovative construction method of voltammetric biosensor based on laccase. Sensors 2018, 18, 4086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanelli, F.; Fracassi, F.; Lapenna, A.; Angarano, V.; Palazzo, G.; Mallardi, A. Atmospheric pressure cold plasma: A friendly environment for dry enzymes. Adv. Mater. Interfaces 2018, 24, 1801373. [Google Scholar] [CrossRef]
- Bahnev, B.; Bowden, M.D.; Stypczyńska, A.; Ptasińska, S.; Mason, N.J.; Braithwaite, N.S.J. A novel method for the detection of plasma jet boundaries by exploring DNA damage. Eur. Phys. J. D 2014, 68, 140. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of atmospheric pressure plasmas on isolated and cellular DNA—A review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, E.R.; Samara, V.; Ptasinska, S. Influence of O2 or H2O in a plasma jet and its environment on plasma electrical and biochemical performances. J. Phys. D 2018, 51, 185202. [Google Scholar] [CrossRef]
- Adhikari, E.R.; Ptasinska, S. Correlation between helium atmospheric pressure plasma jet (APPJ) variables and plasma induced DNA damage. Eur. Phys. J. D 2016, 70, 180. [Google Scholar] [CrossRef]
- Kousal, J.; Shelemin, A.; Kylián, O.; Slavínská, D.; Biederman, H. In-situ monitoring of etching of bovine serum albumin using low-temperature atmospheric plasma jet. Appl. Surf. Sci. 2017, 392, 1049–1054. [Google Scholar] [CrossRef]
- Joslin, J.M.; McCall, J.R.; Bzdek, J.P.; Johnson, D.C.; Hybertson, B.M. Aqueous plasma pharmacy: Preparation methods, chemistry, and therapeutic applications. Plasma Med. 2016, 6, 135–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Ponte, G.; Sardella, E.; Fanelli, F.; Paulussen, S.; Favia, P. Atmospheric pressure plasma deposition of poly lactic acid-like coatings with embedded elastin. Plasma Process. Polym. 2014, 4, 345–352. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Baldus, S.; Steinborn, E.; Edengeiser, E.; Kogelheide, F.; Langklotz, S.; Schneider, S.; Leichert, L.I.O.; Benedikt, J.; Awakowicz, P.; et al. A dielectric barrier discharge terminally inactivates RNase A by oxidizing sulfur-containing amino acids and breaking structural disulfide bonds. J. Phys. D 2015, 48, 494003. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Rezaei, F.; Vanraes, P.; Nikiforov, A.; Morent, R.; De Geyter, N. Applications of plasma-liquid systems: A review. Materials 2019, 12, 2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Borrón, J.C.; Solano, F. Molecular anatomy of tyrosinase and its related proteins: Beyond the histidine-bound metal catalytic center. Pigment Cell Res. 2002, 15, 162–173. [Google Scholar] [CrossRef]
- Halaouli, S.; Asther, M.; Sigoillot, J.C.; Hamdi, M.; Lomascolo, A. Fungal tyrosinases: New prospects in molecular characteristics, bioengineering and biotechnological applications. J. Appl. Microbiol. 2006, 100, 219–232. [Google Scholar] [CrossRef]
- Selinheimo, E.; NiEidhin, D.; Steffensen, C.; Nielsen, J.; Lomascolo, A.; Halaouli, S.; Record, E.; O’Beirne, D.; Buchert, J.; Kruus, K.J. Comparison of the characteristics of fungal and plant tyrosinases. J. Biotechnol. 2007, 130, 471–480. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef]
- Fanelli, F.; Fracassi, F.; d’Agostino, R. Deposition of hydrocarbon films by means of helium-ethylene fed glow dielectric barrier discharges. Plasma Process. Polym. 2005, 2, 688–694. [Google Scholar] [CrossRef]
- Bosso, P.; Fanelli, F.; Fracassi, F. Deposition of water-stable coatings containing carboxylic acid groups by atmospheric pressure cold plasma jet. Plasma Process. Polym. 2016, 13, 217–226. [Google Scholar] [CrossRef]
- Massines, F.; Sarra-Bournet, C.; Fanelli, F.; Naudé, N.; Gherardi, N. Atmospheric pressure low temperature direct plasma technology: Status and challenges for thin film deposition. Plasma Process. Polym. 2012, 9, 1041–1073. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Fanelli, F.; d’Agostino, R.; Fracassi, F. Effect of gas impurities on the operation of dielectric barrier discharges fed with He, Ar, and Ar-C3F6. Plasma Process. Polym. 2012, 9, 1041–1073. [Google Scholar] [CrossRef]
- Rich, S.A.; Dufour, T.; Leroy, P.; Reniers, F.; Nittler, L.; Pireaux, J.J. LDPE surface modifications induced by atmospheric plasma torches with linear and showerhead configurations. Plasma Process. Polym. 2015, 12, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Van Vrekhem, S.; Vloebergh, K.; Asadian, M.; Vercruysse, C.; Declercq, H.; Van Tongel, A.; De Wilde, L.; De Geyter, N.; Morent, R. Improving the surface properties of an UHMWPE shoulder implant with an atmospheric pressure plasma jet. Sci. Rep. 2018, 8, 4720. [Google Scholar] [CrossRef]
- Ionita, E.R.; Ionita, M.D.; Stancu, E.C.; Teodorescu, M.; Dinescu, G. Small size plasma tools for material processing at atmospheric pressure. Appl. Surf. Sci. 2009, 255, 5448–5452. [Google Scholar] [CrossRef]
- Chandrashekaraiah, T.H.; Bogdanowicz, R.; Rühl, E.; Danilov, V.; Meichsner, J.; Thierbach, S.; Hippler, R. Spectroscopic study of plasma polymerized a-C:H films deposited by a dielectric barrier discharge. Materials 2016, 9, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, H.; Polikarpov, I.; Craievich, A.F. Average protein density is a molecular-weight-dependent function. Protein Sci. 2004, 13, 1041–1073. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, F.; Hanus, J.; Kylian, O.; Rossi, F. In situ quartz crystal microbalance measurements of thin protein film plasma removal. Plasma Process. Polym. 2012, 9, 188–196. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-ray photoelectron spectroscopic characterization of chemically modified electrodes used as chemical sensors and biosensors: A review. Sensors 2015, 3, 70–117. [Google Scholar] [CrossRef] [Green Version]







| Feed Mixture | f (kHz) | Va (kVrms) | Ps (W·cm−2) | ΦHe (slm) | [O2] (%) | [C2H4] (%) | t (min) |
|---|---|---|---|---|---|---|---|
| He | 20 | 1.10 | 0.35 ± 0.04 | 8 | - | - | 10, 30, 60 |
| He/O2 | 20 | 1.10 | 0.40 ± 0.04 | 8 | 1.0 | - | 10, 30, 60 |
| He/C2H4 | 20 | 1.10 | 0.40 ± 0.04 | 8 | - | 0.3, 0.5, 1.0 | 30 |
| He/C2H4 | 20 | 0.85 | 0.25 ± 0.05 | 8 | - | 0.3 | 30 |
| He-C2H4 | 20 | 0.85 | 0.25 ± 0.05 | 8 | - | 0.1, 0.3, 0.5 | 10 |
| C at % | O at % | N at % | S at % | P at % | K, Na at % | |
|---|---|---|---|---|---|---|
| Pristine Tyr - control | 64 | 26 | 8.5 | 0.5 | - | 1.0 |
| Tyr - He/1% O2 DBD | 44 | 37 | 7.5 | 1.0 | 3.0 | 7.5 |
| C-C/C-H Peak Area % | C-N/C-O Peak Area % | C=O/N-C=O/O-C-O Peak Area % | COO Peak Area % | |
|---|---|---|---|---|
| 285.0 ± 0.2 eV | 286.3 ± 0.2 eV | 288.1 ± 0.2 eV | 289.0 ± 0.2 eV | |
| Pristine Tyr - control | 37 | 46 | 17 | - |
| Tyr - He/1% O2 DBD | 32 | 41 | 20 | 7 |
| Tyr 2 μg | Tyr 5 μg | GOx 30 μg | GOx 100 μg | |||
|---|---|---|---|---|---|---|
| Feed Mixture | Va (kVrms) | t (min) | Res. Activity (%) | Res. Activity (%) | Res. Activity (%) | Res. Activity (%) |
| He | 1.10 | 30 | 85 ± 4 | 83 ± 4 | 95 ± 6 | 100 ± 6 |
| He/1% O2 | 1.10 | 30 | 70 ± 5 | 67 ± 2 | 14.0 ± 1.0 | 85 ± 2 |
| He/0.3%C2H4 | 1.10 | 30 | - | 84 ± 2 | 96 ± 3 | - |
| He/0.3%C2H4 | 0.85 | 30 | - | 84.0 ± 1.0 | 101 ± 2 | - |
| He/0.3%C2H4 | 0.85 | 10 | - | 100 ± 2 | 100 ± 2 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapenna, A.; Fanelli, F.; Fracassi, F.; Armenise, V.; Angarano, V.; Palazzo, G.; Mallardi, A. Direct Exposure of Dry Enzymes to Atmospheric Pressure Non-Equilibrium Plasmas: The Case of Tyrosinase. Materials 2020, 13, 2181. https://doi.org/10.3390/ma13092181
Lapenna A, Fanelli F, Fracassi F, Armenise V, Angarano V, Palazzo G, Mallardi A. Direct Exposure of Dry Enzymes to Atmospheric Pressure Non-Equilibrium Plasmas: The Case of Tyrosinase. Materials. 2020; 13(9):2181. https://doi.org/10.3390/ma13092181
Chicago/Turabian StyleLapenna, Annamaria, Fiorenza Fanelli, Francesco Fracassi, Vincenza Armenise, Valeria Angarano, Gerardo Palazzo, and Antonia Mallardi. 2020. "Direct Exposure of Dry Enzymes to Atmospheric Pressure Non-Equilibrium Plasmas: The Case of Tyrosinase" Materials 13, no. 9: 2181. https://doi.org/10.3390/ma13092181
APA StyleLapenna, A., Fanelli, F., Fracassi, F., Armenise, V., Angarano, V., Palazzo, G., & Mallardi, A. (2020). Direct Exposure of Dry Enzymes to Atmospheric Pressure Non-Equilibrium Plasmas: The Case of Tyrosinase. Materials, 13(9), 2181. https://doi.org/10.3390/ma13092181