Improving the Properties of Degraded Soils from Industrial Areas by Using Livestock Waste with Calcium Peroxide as a Green Oxidizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection and Preparation
2.3. Physicochemical Analytical Procedures
2.4. Microbiological Analysis
2.5. Reponse Surface Methodology (RSM)
2.6. Phytotest with Grass Seed Mixture
3. Results and Discussion
3.1. Physicochemical and Microbiological Characteristic of Soils and Poultry Manure
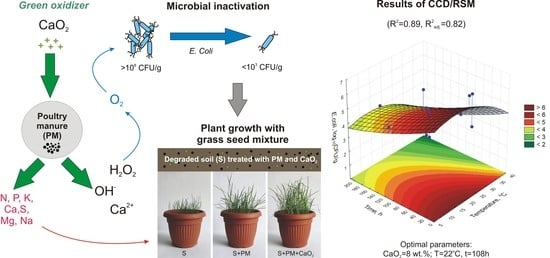
3.2. CCD/RSM Results
3.3. Effect of Inactivation of Poultry Manure Treated with CaO2 on a Grass Seed Mixture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumıtru, M.; Cărăbış, D.; Pârvan, L.; Sârbu, C. Environmental Rehabilitation of Mining Dumps. Agric. Agric. Sci. Procedia 2016, 10, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Haering, K.C.; Lee Daniels, W.; Feagley, S.E. Reclaiming Mined Lands with Biosolids, Manures, and Papermill Sludges. Reclam. Drastically Disturb. Lands 2015, 615–644. [Google Scholar] [CrossRef]
- Sevilla-Perea, A.; Almendros, G.; Mingorance, M.D. Quadratic response models for N and P mineralization in domestic sewage sludge for mininig dump reclamation. Appl. Soil Ecol. 2014, 75, 106–115. [Google Scholar] [CrossRef]
- Dróżdż, D.; Wystalska, K.; Malińska, K.; Grosser, A.; Grobelak, A.; Kacprzak, M. Management of poultry manure in Poland –Current state and future perspectives. J. Environ. Manag. 2020, 264. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.C.; Petrikova, V.; Díaz-Raviña, M.; Carballas, T. Recycling of organic wastes in burnt soils: Combined application of poultry manure and plant cultivation. Waste Manag. 2004, 24, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.C.; Keener, H.M.; Hoitink, H.A.J. Poultry manure composting. An exploratory study. Trans. Am. Soc. Agric. Eng. 1989, 32, 2151–2158. [Google Scholar] [CrossRef]
- Bolan, N.S.; Szogi, A.A.; Chuasavathi, T.; Seshadri, B.; Rothrock, M.J.; Panneerselvam, P. Uses and management of poultry litter. Worlds. Poult. Sci. J. 2010, 66, 673–698. [Google Scholar] [CrossRef] [Green Version]
- Chastain, J.P.; Camberato, J.J.; Skewes, P. Poultry Manure Production and Nutrient Content. In Chapter 3b in: Confined Animal Manure Managers Certification Program Manual B Poultry Version; Clemson University Cooperative Extension Service: Clemson, SC, USA, 2001; Volume 2, pp. 1–17. [Google Scholar]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Bicudo, J.R.; Goyal, S.M. Pathogens and manure management systems: A review. Environ. Technol. 2003, 24, 115–130. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Basamba, T.A. How safe is chicken litter for land application as an organic fertilizer? A review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viegas, C.; Carolino, E.; Malta-Vacas, J.; Sabino, R.; Viegas, S.; Veríssimo, C. Fungal contamination of poultry litter: A public health problem. J. Toxicol. Environ. Health Part A Curr. Issues 2012, 75, 1341–1350. [Google Scholar] [CrossRef] [Green Version]
- Kłapeć, T.; Cholewa, A. Health risk asociated with the use of organic and organic-mineral fertilizers. Gen. Med. Health Sci. 2012, 18, 131–136. [Google Scholar]
- Matusiak, K.; Skóra, J.; Borowski, S.; Pielech-Przybylska, K.; Nowak, A.; Wojewódzki, P.; Hermann, J.; Okrasa, M.; Gutarowska, B. Threats/risk in poultry farms: Microbiological Contaminants, dust, odours and biological method for elimination. Ecol. Eng. 2017, 18, 184–193. [Google Scholar] [CrossRef]
- Irshad, M.; Malik, A.H.; Shaukat, S.; Mushtaq, S.; Ashraf, M. Characterization of Heavy Metals in Livestock Manures. Polish J. Environ. Stud. 2013, 22, 1257–1262. [Google Scholar]
- Zhao, X.; Wang, J.; Zhu, L.; Ge, W.; Wang, J. Environmental analysis of typical antibiotic-resistant bacteria and ARGs in farmland soil chronically fertilized with chicken manure. Sci. Total Environ. 2017, 593–594, 10–17. [Google Scholar] [CrossRef]
- Diez-Gonzalez, F.; Jarvis, G.N.; Adamovich, D.A.; Russell, J.B. Use of carbonate and alkali to eliminate Escherichia coli from dairy cattle manure. Environ. Sci. Technol. 2000, 34, 1275–1279. [Google Scholar] [CrossRef]
- Unc, A.; Goss, M.J. Transport of bacteria from manure and protection of water resources. Appl. Soil Ecol. 2004, 25, 1–18. [Google Scholar] [CrossRef]
- Minister of Agriculture and Rural Development. Regulation of 18 June 2008 on the implementation of certain provisions of fertilizers and fertilization. J. Low Pol. 2008, 119, 6515–6520. [Google Scholar]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.; O’Dwyer, T.; Sutton, D.; Leahy, M. Advances in poultry litter disposal technology—A review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef]
- Larney, F.J.; Yanke, L.J.; Miller, J.J.; McAllister, T.A. Fate of coliform bacteria in composted beef cattle feedlot manure. J. Environ. Qual. 2003, 32, 1508–1515. [Google Scholar] [CrossRef]
- Heinonen-Tanski, H.; Mohaibes, M.; Karinen, P.; Koivunen, J. Methods to reduce pathogen microorganisms in manure. Livest. Sci. 2006, 102, 248–255. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Richard, T.L.; Honeyman, M.S. Effect of windrow turning and seasonal temperatures on composting of hog manure from hoop structures. Environ. Technol. 2000, 21, 1037–1046. [Google Scholar] [CrossRef]
- Practical Guidelines for Disinfection with Lime, 3rd ed.; European Lime Association: Brussels, Belgium, 2009.
- Walawska, B.; Gluziłska, J.; Miksch, K.; Turek-Szytow, J. Solid inorganic peroxy compounds in environmental protection. Polish J. Chem. Technol. 2007, 9, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Waite, A.; Bonner, J.; Autenrieth, R. Kinetics and from Solid Peroxides. Environ. Eng. Sci. 1999, 16, 187–199. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Li, T.; Chen, Z.; Wang, Y.; Qin, C. Properties of calcium peroxide for release of hydrogen peroxide and oxygen: A kinetics study. Chem. Eng. J. 2016, 303, 450–457. [Google Scholar] [CrossRef]
- Cassidy, D.P.; Irvine, R.L. Use of calcium peroxide to provide oxygen for contaminant biodegradation in a saturated soil. J. Hazard. Mater. 1999, 69, 25–39. [Google Scholar] [CrossRef]
- Cho, I.; Lee, K. Effect of calcium peroxide on the growth and proliferation of Microcystis aerusinosa, a water-blooming cyanobacterium. Biotechnol. Bioprocess Eng. 2002, 7, 231–233. [Google Scholar] [CrossRef]
- Domaradzki, M.; Kaniewska, J.; Korpal, W. Oxygen fertilizers in technology of plant seeds. Influence of CaO2 additive on the quality of pelleted seeds. Chemik 2012, 66, 464–466. [Google Scholar]
- Lu, S.; Zhang, X.; Xue, Y. Application of calcium peroxide in water and soil treatment: A review. J. Hazard. Mater. 2017, 337, 163–177. [Google Scholar] [CrossRef]
- López, D.A.R.; Mueller, D. Use of calcium peroxide in bioremediation of soils contamined with hydocarbons. Cad. Pesqui. Ser. Biol. 2009, 21, 61–72. [Google Scholar]
- Northup, A.; Cassidy, D. Calcium peroxide (CaO2) for use in modified Fenton chemistry. J. Hazard. Mater. 2008, 152, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Goi, A.; Viisimaa, M.; Trapido, M.; Munter, R. Polychlorinated biphenyls-containing electrical insulating oil contaminated soil treatment with calcium and magnesium peroxides. Chemosphere 2011, 82, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, J.; Li, Y. Performance of calcium peroxide for removal of endocrine-disrupting compounds in waste activated sludge and promotion of sludge solubilization. Water Res. 2015, 71, 125–139. [Google Scholar] [CrossRef]
- Małachowska-Jutsz, A.; Turek-Szytow, J.; Miksch, K. Effect of calcium peroxide on zootoxity in fluoranthene-contaminated soil. Przem. Chem. 2014, 2197–2200. [Google Scholar] [CrossRef]
- Syu, C.H.; Yu, C.H.; Lee, D.Y. Effect of applying calcium peroxide on the accumulation of arsenic in rice plants grown in arsenic-elevated paddy soils. Environ. Pollut. 2020, 266, 115140. [Google Scholar] [CrossRef]
- Fu, S.F.; Chen, K.Q.; Zou, H.; Xu, J.X.; Zheng, Y.; Wang, Q.F. Using calcium peroxide (CaO2) as a mediator to accelerate tetracycline removal and improve methane production during co-digestion of corn straw and chicken manure. Energy Convers. Manag. 2018, 172, 588–594. [Google Scholar] [CrossRef]
- Turek-Szytow, J.; Marciocha, D.; Kalka, J.; Surmacz-Górska, J. Peroxide impact on the fate of veterinary drugs in fertilizers. Chem. Pap. 2020, 74, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons: New York, NY, USA, 2016; ISBN 9781118916032. [Google Scholar]
- Sikder, S.; Joardar, J.C. Biochar production from poultry litter as management approach and effects on plant growth. Int. J. Recycl. Org. Waste Agric. 2019, 8, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.; Slater, F.M. The effects of organic and inorganic fertilizer applications to Miscanthus×giganteus, Arundo donax and Phalaris arundinacea, when grown as energy crops in Wales, UK. Glob. Chang. Biol. Bioenergy 2010, 2, 169–179. [Google Scholar] [CrossRef]
- Joardar, J.C.; Rahman, M.M. Poultry feather waste management and effects on plant growth. Int. J. Recycl. Org. Waste Agric. 2018, 7, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Tripathi, D.; Singh, V.; Chauhan, D.; Prasad, S.; Dubey, N. Improvement of Crops in the Era of Climatic Changes; Springer: New York, NY, USA, 2014; Volume 2, ISBN 9781461488248. [Google Scholar]
- Ahmad, P.; Wani, M.R.; Azooz, M.M.; Phan Tran, L.S. Improvement of crops in the era of climatic changes. Improv. Crop. Era Clim. Chang. 2014, 2, 1–368. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Jacobsen, J. Plant Nutrient Functions and Deficiency and Toxicity Symptoms. Nutr. Manag. Modul. 2011, 9, 1–16. [Google Scholar]
- Ghaly, A.E.; Alhattab, M. Drying poultry manure for pollution potential reduction and production of organic fertilizer. Am. J. Environ. Sci. 2013, 9, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Wieckol-Ryk, A.; Bialecka, B.; Thomas, M. Application of calcium peroxide as an environmentally friendly oxidant to reduce pathogens in organic fertilizers and its impact on phosphorus bioavailability. Arch. Environ. Prot. 2020, 46, 42–53. [Google Scholar] [CrossRef]
- Mituniewicz, T.; Piotrowska, J.; Sowińska, J.; Mituniewicz, E.; Iwańczuk-Czernik, K.; Wójcik, A. Effect of calcium peroxide (CaO2) addition to poultry litter on the parameters of its physicochemical, microbiological and fertilising quality. J. Elem. 2016, 21, 1327–1341. [Google Scholar] [CrossRef]
- Sladdin, M.; Lynch, J. Effect of calcium peroxide, lime and other seed dressings on winter wheat establishment under wet conditions. Crop Prot. 1983, 2, 113–119. [Google Scholar] [CrossRef]
- Le Thi, P.; Lee, Y.; Tran, D.L.; Hoang Thi, T.T.; Park, K.M.; Park, K.D. Calcium peroxide-mediated: In situ formation of multifunctional hydrogels with enhanced mesenchymal stem cell behaviors and antibacterial properties. J. Mater. Chem. B 2020, 8, 11033–11043. [Google Scholar] [CrossRef]
- Thomas, M.; Zdebik, D. Treatment of real textile wastewater by using potassium ferrate(VI) and fe(III)/H2O2. application of aliivibrio fischeri and brachionus plicatilis tests for toxicity assessment. Fibres Text. East. Eur. 2019, 27, 78–84. [Google Scholar] [CrossRef]
- Kozik, V.; Barbusinski, K.; Thomas, M.; Sroda, A.; Jampilek, J.; Sochanik, A.; Smolinski, A.; Bak, A. Taguchi Method and Response Surface Methodology in the Treatment of Highly Contaminated Tannery Wastewater Using Commercial Potassium Ferrate. Materials 2019, 12, 3784. [Google Scholar] [CrossRef] [Green Version]
- Kwak, T.Y.; Kim, N.H.; Rhee, M.S. Response surface methodology-based optimization of decontamination conditions for Escherichia coli O157:H7 and Salmonella Typhimurium on fresh-cut celery using thermoultrasound and calcium propionate. Int. J. Food Microbiol. 2011, 150, 128–135. [Google Scholar] [CrossRef]
- M-Ridha, M.J.; Hussein, S.I.; Alismaeel, Z.T.; Atiya, M.A.; Aziz, G.M. Biodegradation of reactive dyes by some bacteria using response surface methodology as an optimization technique. Alexandria Eng. J. 2020, 59, 3551–3563. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, T.; Doyle, M.P. Fate of enterohemorrhagic Escherichia coli O157:H7 in bovine feces. Appl. Environ. Microbiol. 1996, 62, 2567–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paluszak, Z.; Ligocka, A.; Breza-Boruta, B.; Olszewska, H. The survival of selected fecal bacteria in peat soil amended with slurry. Electron. J. Polish Agric. Univ. 2003, 6. [Google Scholar]
- Gerba, C.P.; Smith, J.E. Sources of pathogenic microorganisms and their fate during land application of wastes. J. Environ. Qual. 2005, 34, 42–48. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Chekabab, S.M.; Paquin-Veillette, J.; Dozois, C.M.; Harel, J. The ecological habitat and transmission of Escherichia coli O157:H7. FEMS Microbiol. Lett. 2013, 341, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-S.; Heu, S.-G.; Roh, E.-J.; Kim, M.-H.; Gil, H.-J.; Choi, N.-Y.; Lee, D.-H.; Lim, J.-A.; Ryu, J.-G.; Kim, K.-H. Effect of Temperature on Survival of Escherichia coli O157:H7 and Listeria monocytogenes in Livestock Manure Compost. Korean J. Soil Sci. Fertil. 2013, 46, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Morgan, J.; Doyle, M.P. Fate of Escherichia coli O157:H7 in Manure-Amended Soil. Appl. Environ. Microbiol. 2002, 68, 2605–2609. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.M.; Hatton, W. Calcium peroxide as a seed coating material for padi rise. Plant Soil 1987, 99, 357–363. [Google Scholar] [CrossRef]
- Mei, J.; Wang, W.; Peng, S.; Nie, L. Seed Pelleting with Calcium Peroxide Improves Crop Establishment of Direct-seeded Rice under Waterlogging Conditions. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, J.K.; Ando, H.; Kakuda, K.I.; Purwanto, B.H. Effect of calcium peroxide coating, soil source, and genotype on rice (Oryza sativa L.) seedling establishment under hypoxic conditions. Soil Sci. Plant. Nutr. 2001, 47, 477–488. [Google Scholar] [CrossRef] [Green Version]
- Javed, T.; Afzal, I.; Mauro, R.P. Seed coating in direct seeded rice: An innovative and sustainable approach to enhance grain yield and weed management under submerged conditions. Sustainability 2021, 13, 2190. [Google Scholar] [CrossRef]






| Parameter | Unit | PM | S1 | S2 |
|---|---|---|---|---|
| Moisture content | % | 62.5 | 0.6 | 0.6 |
| Ash content | % | 12.9 | 95.4 | 97.5 |
| TOC | g/kg dm | 418.5 | nd | nd |
| S | 6.2 | 0.3 | 0.3 | |
| N | 56.7 | nd | nd | |
| P | 20.1 | 0.3 | 0.2 | |
| K | 23.6 | 7.2 | 4.8 | |
| Ca | 24.0 | 1.6 | 0.6 | |
| Mg | 8.5 | 0.7 | 0.2 | |
| Na | 5.3 | 0.8 | 0.1 | |
| Si | 1.8 | 413.8 | 441.8 | |
| Al | 0.5 | 15.5 | 9.5 | |
| Fe | 0.8 | 11.0 | 3.7 | |
| Ba | mg/kg dm | 370.0 | 308.8 | 201.2 |
| Cd | bdl | 35.2 | 12.1 | |
| Co | bdl | 3.0 | 5.0 | |
| Cr | bdl | 12.1 | 13.1 | |
| Cu | 68.0 | 42.3 | 17.1 | |
| Mn | 383.0 | 434.5 | 133.8 | |
| Ni | 20.0 | 10.1 | 7.0 | |
| Pb | bdl | 942.5 | 387.2 | |
| Rb | 13.0 | 94.6 | 30.2 | |
| Sr | 34.0 | 3.0 | 4.0 | |
| Zn | 428.0 | 6920.1 | 746.3 | |
| E. coli | log10CFU/g | 8.3 | nd | nd |
| Run | Experimental Conditions | Experimental Results | ||
|---|---|---|---|---|
| CaO2 (wt %) | Temperature (°C) | Contact Time (h) | E. coli (log10 CFU/g) | |
| 1 | 3.0 | 12.0 | 48 | 6.2304 |
| 2 | 3.0 | 12.0 | 168 | 5.1761 |
| 3 | 3.0 | 32.0 | 48 | 5.7782 |
| 4 | 3.0 | 32.0 | 168 | 4.6435 |
| 5 | 7.0 | 12.0 | 48 | 4.5441 |
| 6 | 7.0 | 12.0 | 168 | 3.9777 |
| 7 | 7.0 | 32.0 | 48 | 3.4771 |
| 8 | 7.0 | 32.0 | 168 | 3.0000 |
| 9 | 1.6 | 22.0 | 108 | 7.8451 |
| 10 | 8.4 | 22.0 | 108 | 3.0000 |
| 11 | 5.0 | 5.2 | 108 | 5.0414 |
| 12 | 5.0 | 38.8 | 108 | 3.0000 |
| 13 | 5.0 | 22.0 | 7 | 6.9868 |
| 14 | 5.0 | 22.0 | 209 | 3.2788 |
| 15 (C) * | 5.0 | 22.0 | 108 | 4.9085 |
| 16 (C) * | 5.0 | 22.0 | 108 | 4.6435 |
| Parameter | The Evaluation of the Effects, E. Coli log10CFU/g; R2 = 0.89008, R2adj = 0.8168, 3 Parameters, 1 Block, 16 Experiments, MS = 0.3877 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Standard Error | p-Value * | −95% Confidence Interval | +95% Confidence Interval | Factor | Standard Error of Factor | Lower Confidence Interval | Upper Confidence Interval | |
| Constant value | 4.80110 | 0.439007 | 0.000002 | 3.80799 | 5.79420 | 4.80110 | 0.439007 | 3.80799 | 5.794202 |
| CaO2, wt % (L) | −2.19344 | 0.336989 | 0.000110 | −2.95576 | −1.43112 | −1.09672 | 0.168495 | −1.47788 | −0.715559 |
| CaO2, wt % (Q) | 0.33599 | 0.409156 | 0.432751 | −0.58958 | 1.26157 | 0.16800 | 0.204578 | −0.29479 | 0.630785 |
| Temperature, °C (L) | −0.94644 | 0.336989 | 0.020426 | −1.70877 | −0.18412 | −0.47322 | 0.168495 | −0.85438 | −0.092060 |
| Temperature, °C (Q) | −0.65526 | 0.409156 | 0.143731 | −1.58084 | 0.27031 | −0.32763 | 0.204578 | −0.79042 | 0.135156 |
| Contact time, h (L) | −1.38691 | 0.336989 | 0.002615 | −2.14924 | −0.62459 | −0.69346 | 0.168495 | −1.07462 | −0.312295 |
| Contact time, h (Q) | 0.13072 | 0.409156 | 0.756645 | −0.79485 | 1.05630 | 0.06536 | 0.204578 | −0.39743 | 0.528149 |
| Parameter | Assessment of the Effects, E. Coli log10CFU/g; R2 = 0.89008, R2adj = 0.8168, 3 Parameters, 1 Block, 16 Experiments, MS = 0.3877 | ||||
|---|---|---|---|---|---|
| SS | DF | MS | F | p-Value * | |
| CaO2, wt % (L) | 16.4264 | 1 | 16.4264 | 42.3662 | 0.0001 |
| CaO2, wt % (Q) | 0.2615 | 1 | 0.2615 | 0.6744 | 0.4328 |
| Temperature, °C (L) | 3.0583 | 1 | 3.0583 | 7.8878 | 0.0204 |
| Temperature, °C (Q) | 0.9944 | 1 | 0.9944 | 2.5648 | 0.1437 |
| Contact time, h (L) | 6.5673 | 1 | 6.5673 | 16.9382 | 0.0026 |
| Contact time, h (Q) | 0.0396 | 1 | 0.0396 | 0.1021 | 0.7566 |
| Error | 3.4895 | 9 | 0.3877 | - | - |
| Predictor | Regression Coefficient | Standard Error | t-Value, df * = 9 | p-Value ** | −95% Confidence Interval | +95% Confidence Interval |
|---|---|---|---|---|---|---|
| Intercept | 9.508227 | 2.024962 | 4.69551 | 0.001127 | 4.92744 | 14.08901 |
| CaO2 (L) | −0.968354 | 0.518337 | −1.86819 | 0.094571 | −2.14091 | 0.20421 |
| CaO2 (Q) | 0.041999 | 0.051145 | 0.82119 | 0.432751 | −0.07370 | 0.15770 |
| Temperature (L) | 0.096836 | 0.091578 | 1.05741 | 0.317877 | −0.11033 | 0.30400 |
| Temperature (Q) | −0.003276 | 0.002046 | −1.60150 | 0.143731 | −0.00790 | 0.00135 |
| Time (L) | −0.015479 | 0.012592 | −1.22931 | 0.250124 | −0.04396 | 0.01301 |
| Time (Q) | 0.000018 | 0.000057 | 0.31949 | 0.756645 | −0.00011 | 0.00015 |
| Soil Sample | Average Lenght of Root * (cm) | Average Lenght of Shoot * (cm) | Weigh of Fresh Biomass ** (g) | Weigh of Dried Biomass ** (g) | GFR (%) | GFS (%) |
|---|---|---|---|---|---|---|
| S1 | 0.15 ± 0.08 | 4.99 ± 1.19 | 0.447 ± 0.050 | 0.095 ± 0.010 | - | - |
| S1 + 1% PM | 0.68 ± 0.30 | 7.83 ± 1.64 | 0.966 ± 0.070 | 0.187 ± 0.030 | 77.94 | 36.27 |
| S1 + 1% PM + B | 1.03 ± 0.32 | 8.78 ± 1.72 | 1.790 ± 0.080 | 0.292 ± 0.020 | 85.44 | 43.17 |
| S1 + 2% PM | 0.72 ± 0.27 | 7.98 ± 1.45 | 1.194 ± 0.060 | 0.231 ± 0.030 | 79.37 | 37.47 |
| S1 + 2% PM + B | 2.82 ± 1.05 | 10.32 ± 1.73 | 1.837 ± 0.070 | 0.346 ± 0.030 | 94.68 | 51.65 |
| S2 | 0.20 ± 0.12 | 5.93 ± 1.31 | 0.879 ± 0.040 | 0.201 ± 0.020 | - | - |
| S2 + 1%PM | 1.91 ± 0.66 | 10.34 ± 2.09 | 2.221 ± 0.050 | 0.370 ± 0.030 | 89.51 | 42.67 |
| S2 + 1%PM+B | 4.58 ± 1.44 | 11.26 ± 1.66 | 2.869 ± 0.070 | 0.445 ± 0.040 | 95.63 | 47.33 |
| S2 + 2%PM | 2.55 ± 0.78 | 10.81 ± 1.47 | 2.229 ± 0.060 | 0.316 ± 0.020 | 92.16 | 45.14 |
| S2 + 2%PM+B | 2.89 ± 0.75 | 11.84 ± 1.44 | 2.811 ± 0.090 | 0.415 ± 0.050 | 93.08 | 49.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Więckol-Ryk, A.; Thomas, M.; Białecka, B. Improving the Properties of Degraded Soils from Industrial Areas by Using Livestock Waste with Calcium Peroxide as a Green Oxidizer. Materials 2021, 14, 3132. https://doi.org/10.3390/ma14113132
Więckol-Ryk A, Thomas M, Białecka B. Improving the Properties of Degraded Soils from Industrial Areas by Using Livestock Waste with Calcium Peroxide as a Green Oxidizer. Materials. 2021; 14(11):3132. https://doi.org/10.3390/ma14113132
Chicago/Turabian StyleWięckol-Ryk, Angelika, Maciej Thomas, and Barbara Białecka. 2021. "Improving the Properties of Degraded Soils from Industrial Areas by Using Livestock Waste with Calcium Peroxide as a Green Oxidizer" Materials 14, no. 11: 3132. https://doi.org/10.3390/ma14113132
APA StyleWięckol-Ryk, A., Thomas, M., & Białecka, B. (2021). Improving the Properties of Degraded Soils from Industrial Areas by Using Livestock Waste with Calcium Peroxide as a Green Oxidizer. Materials, 14(11), 3132. https://doi.org/10.3390/ma14113132