Opportunities, Challenges and Prospects for Electrodeposition of Thin-Film Functional Layers in Solid Oxide Fuel Cell Technology
Abstract
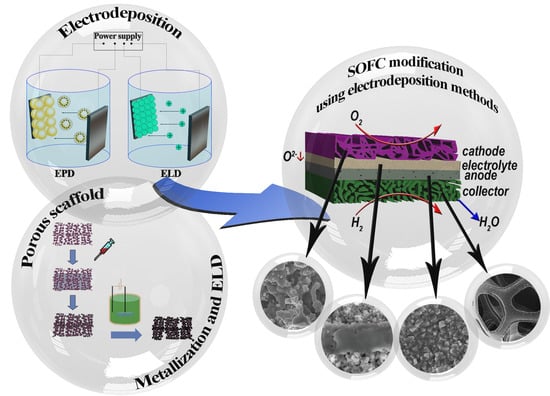
:1. Introduction
2. Electrodeposition Technology for the Fabrication of SOFC Air Electrodes with Increased Performance
2.1. Electrochemical Synthesis Techniques
2.2. Fabrication of Composite Air Electrodes Based on Perovskite Electrode Materials Utilizing Electrodeposition Methods
2.3. Electrodeposition of the Composite Electrodes Based on Porous Pt and Ag
2.4. Electrodeposition of the Electrodes with Pore-Formers and Development of Oriented Structures
3. Electrodeposition Methods for the Formation of SOFC Ni-Cermet Anodes and Modification of Ni-Foam-Based Anode Collectors
4. Formation of Films Based on Cerium Dioxide by Electrodeposition
4.1. Cathodic ELD

4.2. Anodic ELD
4.3. Electrophoretic Deposition of CeO2-Based Thin-Film Electrolyte Membranes

5. Environmental and Economic Aspects of the Application of Electrodeposition in SOFC Technology
6. Conclusions
- -
- Difficulties associated with ELD of multicomponent coatings;
- -
- The complexity of identifying the structure and phase content of the resulting coatings;
- -
- High sensitivity to the conditions and modes of electrodeposition and non-reproducibility of results in some cases;
- -
- The formation of loose deposits and the formation of cracks and pores in the CeO2-based coatings obtained using cathodic ELD. The problem of cracking of electrodeposited coatings can be partially solved by co-deposition with a binder (for example, PDDA) or by the electrochemical synthesis of films of doped cerium dioxide;
- -
- The challenges in forming the multilayer and composite coatings;
- -
- The need to create the surface electrical conductivity of the non-conductive substrate for the implementation of the electrodeposition process;
- -
- The necessity of loweing the sintering temperature of the EPD coatings to preserve the porosity of the carrying substrates (SOFC electrodes).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pikalova, E.Y.; Kalinina, E.G. Electrophoretic deposition in the solid oxide fuel cell technology: Fundamentals and recent advances. Renew. Sustain. Energy Rev. 2019, 116, 109440. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Petric, A. The electrodeposition of ceramic and organoceramic films for fuel cells. JOM 2001, 53, 48–50. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Aznam, I.; Mah, J.C.W.; Muchtar, A.; Samalu, M.R.; Ghazali, M.J. A review of key parameters for effective electrophoretic deposition in the fabrication of solid oxide fuel cells. J. Zhejiang Univ. Sci. A 2018, 19, 811–823. [Google Scholar] [CrossRef]
- Das, D.; Basu, R.N. Electrophoretically deposited thin film electrolyte for solid oxide fuel cell. Adv. Appl. Ceram. 2014, 113, 8–13. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques. Int. J. Hydrog. Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Lee, K.T.; Wachsman, E.D. Role of nanostructures on SOFC performance at reduced temperatures. MRS Bull. 2014, 39, 783–791. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Solid oxide fuel cells based on ceramic membranes with mixed conductivity: Improving efficiency. Russ. Chem. Rev. 2021, 90, 703–749. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Zhang, Y.; Wang, R.; Ouyang, Z.; Gou, Z.; Zhang, H. Recent advances in two-dimensional materials and their nanocomposites in sustainable energy conversion applications. Nanoscale 2019, 11, 21622–21678. [Google Scholar] [CrossRef]
- Ahmad, K.; Ahmad, M.A.; Raza, R.; Khan, M.A.; Rehman, Z.U.; Abbas, G. Graphene Incorporated Nanocomposite Anode for Low Temperature SOFCs. J. Electron. Mater. 2019, 48, 7507–7514. [Google Scholar] [CrossRef]
- Jee, Y.; Karimaghaloo, A.; Andrade, A.M.; Moon, H.; Li, Y.; Han, J.W.; Lee, M.H. Graphene-based Oxygen Reduction Electrodes for Low Temperature Solid Oxide Fuel Cells. Fuel Cells 2017, 17, 344–352. [Google Scholar] [CrossRef]
- Hakamada, M.; Moriguchi, A.; Mabuchi, M. Fabrication of carbon nanotube/NiOx(OH)y nanocomposite by pulsed electrodeposition for supercapacitor applications. J. Power Sources 2014, 245, 324–330. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Yang, Y.; Cristian, M.S.; Wang, J.; Hou, X.; Yan, B.; Zhang, T.; Paillard, E.; Swietoslawski, M.; Kostecki, R.; et al. Uniform lithium electrodeposition for stable lithium-metal batteries. Nano Energy 2019, 67, 104172. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, H.; Zheng, F.; Huang, T.; Wu, F.; Wang, H. Electrodeposition of graphene by cyclic voltammetry on nickel electrodes for microbial fuel cells applications. Int. J. Energy Res. 2019, 43, 2795–2805. [Google Scholar] [CrossRef]
- Rehman, M.A.; Chen, Q.; Braem, A.; Shaffer, M.S.P.; Boccaccini, A.R. Electrophoretic deposition of carbon nanotubes: Recent progress and remaining challenges. Int. Mater. Rev. 2020, 1–30. [Google Scholar] [CrossRef]
- She, G.; Mu, L.; Shi, W. Electrodeposition of One-Dimensional Nanostructures. Recent Pat. Nanotechnol. 2009, 3, 182–191. [Google Scholar] [CrossRef]
- Vinokurov, E.G.; Margolin, L.N.; Farafonov, V.V. Electrodeposition of composite coatings. Izv. Vuz. Khim. Kh. Tekh. 2020, 63, 4–38. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Place of electrophoretic deposition among thin-film methods adapted to the solid oxide fuel cell technology: A short review. Int. J. Energy Sect. Manag. 2019, 4, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Zhitomirsky, I. Cathodic electrodeposition of cermic and organoceramic materials. Fundamental aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Ata, M.S.; Zhang, T.; Wallar, C.J.; Zhitomirsky, I. Universal dispersing agent for electrophoretic deposition of inorganic materials with improved adsorption, triggered by chelating monomers. J. Colloid Interface Sci. 2016, 462, 1–8. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Petric, A. Electrophoretic deposition of ceramic materials for fuel cell applications. J. Eur. Ceram. Soc. 2000, 20, 2055–2061. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y. New trends in the development of electrophoretic deposition method in the solid oxide fuel cell technology: Theoretical approaches, experimental solutions and development prospects. Russ. Chem. Rev. 2019, 88, 1179–1219. [Google Scholar] [CrossRef]
- Burmistrov, I.N.; Agarkov, D.A.; Tsybrov, F.M.; Bredikhin, S.I. Preparation of membrane-electrode assemblies of solid oxide fuel cells by co-sintering of electrodes. Russ. J. Electrochem. 2016, 52, 669–677. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Lyagaeva, Y.G.; Vylkov, A.I.; Gorshkov, M.Y.; Vdovin, G.K.; Medvedev, D.A. Performance of Pr2(Ni,Cu)O4+δ electrodes in protonic ceramic electrochemical cells with unseparated and separated gas spaces. J. Mater. Sci. Technol. 2021, 93, 157–168. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Sadykov, V.A.; Filonova, E.A.; Eremeev, N.F.; Sadovskaya, E.M.; Pikalov, S.M.; Bogdanovich, N.M.; Lyagaeva, J.G.; Kolchugin, A.A.; Vedmid’, L.B.; et al. Structure, oxygen transport properties and electrode performance of Ca-substituted Nd2NiO4. Solid State Ion. 2019, 335, 53–60. [Google Scholar] [CrossRef]
- Banitaba, S.N.; Ehrmann, A. Application of Electrospun Nanofibers for Fabrication of Versatile and Highly Efficient Electrochemical Devices: A Review. Polymers 2021, 13, 1741. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Zhang, H.; Li, C.; Zhang, S.; Yang, G.; Li, C. Structured La0.6Sr0.4Co0.2Fe0.8O3–δ cathode with large-scale vertical cracks by atmospheric laminar plasma spraying for IT-SOFCs. J. Alloy. Compd. 2020, 825, 153865. [Google Scholar] [CrossRef]
- Develos-Bagarinao, K.; Budiman, R.A.; Ishiyama, T.; Yamaji, K.; Kishimoto, H. Leveraging catalytic effects of heterointerfaces through multilayering for superior cathode performance. J. Power Sources 2020, 480, 229094. [Google Scholar] [CrossRef]
- Sase, M.; Hermes, F.; Yashiro, K.; Sato, K.; Mizusaki, J.; Kawada, T.; Sakai, N.; Yokokawa, H. Enhancement of Oxygen Surface Exchange at the Hetero-interface of ( La,Sr)CoO3/( La,Sr)2CoO4 with PLD-Layered Films. J. Electrochem. Soc. 2008, 155, B793–B797. [Google Scholar] [CrossRef]
- Liu, Y.; Compson, C.; Liu, M. Nanostructured and functionally graded cathodes for intermediate-temperature SOFCs. Fuel Cells Bull. 2004, 2004, 12–15. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Finklea, H.; Liu, X. A review of electrophoretic deposition of metal oxides and its application in solid oxide fuel cells. Adv. Colloid Interface Sci. 2020, 276, 102102. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, Y.; Watanabe, S.; Yamaji, T.; Asamoto, M.; Yahiro, H.; Sadaoka, Y. Electrophoretic deposition of bi-layered LSM/LSM-YSZ cathodes for solid oxide fuel cell. J. Power Sources 2012, 214, 153–158. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamat, P.V. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsumoto, Y.; Hombo, J.; Ogawa, Y. A new preparation method of LaMnO3 perovskite using electrochemical oxidation. J. Solid State Chem. 1991, 91, 61–70. [Google Scholar] [CrossRef]
- Sasaki, T.; Morikawa, T.; Hombo, J.; Matsumoto, M. Electrochemical Synthesis of La1-xSrxMnO3 Perovskite Films. Denki Kagaku 1990, 58, 567–568. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Sasaki, T.; Hombo, J. Effect of La3+ ions on the deposition of nickel oxide. Electrochim. Acta 1993, 38, 1145–1146. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sasaki, T.; Hombo, J. A new preparation method of lanthanum cobalt oxide, LaCoO3, perovskite using electrochemical oxidation. Inorg. Chem. 1992, 31, 738–741. [Google Scholar] [CrossRef]
- Enache, S.; Dragan, M.; Varlam, M.; Petrov, K. Electronic Percolation Threshold of Self-Standing Ag-LaCoO3 Porous Electrodes for Practical Applications. Materials 2019, 12, 2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepech-Carrillo, L.; Escobedo-Morales, A.; Pérez-Centeno, A.; Chigo-Anota, E.; Sánchez-Ramírez, J.F.; López-Apreza, E.; Gutiérrez-Gutiérrez, J. Preparation of Nanosized LaCoO3 through Calcination of a Hydrothermally Synthesized Precursor. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Therese, G.H.A.; Kamath, P.V. Electrochemical Synthesis of LaMnO3 Coatings on Conducting Substrates. Chem. Mater. 1998, 10, 3364–3367. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Dinamani, M.; Vishnu Kamath, P. Electrochemical synthesis of perovskite oxides. J. Appl. Electrochem. 2005, 35, 459–465. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamath, P.V. Electrochemical Synthesis of Ln2Cr3O12·7H2O (Ln = La, Pr, Nd). Mater. Res. Bull. 1998, 33, 1–7. [Google Scholar] [CrossRef]
- Konno, H.; Tokita, M.; Furusaki, A.; Furuichi, R. Electrochemical formation of a-site substituted perovskite structure La1−xMxCrO3 oxide coatings. Electrochim. Acta 1992, 37, 2421–2426. [Google Scholar] [CrossRef]
- Shi, Y.; Meng, H.; Sun, D.; Yu, H.; Fu, H. Manganese Oxide Coating Electrodes Prepared by Pulse Anodic Electrodeposition. Acta Phys. Chim. Sin. 2008, 24, 1199–1206. [Google Scholar] [CrossRef]
- Kireev, S.Y. Intensification of processes of electrodeposition of metals by use of various modes of pulse electrolysis. Inorg. Mater. 2017, 8, 203–210. [Google Scholar] [CrossRef]
- Kireev, S.Y.; Perelygin, Y.P.; Kireeva, S.N.; Jaskula, M.J. Methods to Determine the Current Efficiency in AC Electrolysis. Arab. J. Sci. Eng. 2021, 46, 343–352. [Google Scholar] [CrossRef]
- Jiang, Z.; Xia, C.; Chen, F. Nano-structured composite cathodes for intermediate-temperature solid oxide fuel cells via an infiltration/impregnation technique. Electrochim. Acta 2010, 55, 3595–3605. [Google Scholar] [CrossRef]
- Chen, D.; He, H.; Zhang, D.; Wang, H.; Ni, M. Percolation Theory in Solid Oxide Fuel Cell Composite Electrodes with a Mixed Electronic and Ionic Conductor. Energies 2013, 6, 1632–1656. [Google Scholar] [CrossRef] [Green Version]
- Bidrawn, F.; Vohs, J.M.; Gorte, R.J. Fabrication of LSM–YSZ Composite Electrodes by Electrodeposition. J. Electrochem. Soc. 2010, 157, B1629–B1633. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Oh, T.S.; Li, Y.; Vohs, J.M.; Gorte, R.J. Fabrication of MnCo2O4-YSZ Composite Cathodes for Solid Oxide Fuel Cells by Electrodeposition. J. Electrochem. Soc. 2016, 163, F863–F866. [Google Scholar] [CrossRef]
- Pinto, R.; Carmezim, M.J.; Montemor, M.F. Electrodeposition and isothermal aging of Co and Mn layers on stainless steel for interconnectors: Initial stages of spinel phase formation. J. Power Sources 2014, 255, 251–259. [Google Scholar] [CrossRef]
- Rehman, S.U.; Song, R.; Lim, T.H.; Park, S.J.; Hong, J.E.; Lee, J.W.; Lee, S.B. High-performance nanofibrous LaCoO3 perovskite cathode for solid oxide fuel cells fabricated via chemically assisted electrodeposition. J. Mater. Chem. A 2018, 6, 6987–6996. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shaur, A.; Song, R.H.; Lim, T.H.; Hong, J.E.; Park, S.J.; Lee, S.B. Nano-fabrication of a high-performance LaNiO3 cathode for solid oxide fuel cells using an electrochemical route. J. Power Sources 2019, 429, 97–104. [Google Scholar] [CrossRef]
- Shaur, A.; Rehman, S.U.; Kim, H.S.; Song, R.H.; Lim, T.H.; Hong, J.E.; Park, S.J.; Lee, S.B. Hybrid electrochemical deposition route for the facile nanofabrication of Cr-poisoning-tolerant La(Ni,Fe)O3–δ cathode for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 5730–5738. [Google Scholar] [CrossRef]
- Ai, N.; Li, N.; Rickard, W.D.A.; Cheng, Y.; Chen, K.; Jiang, S.P. Highly Stable Sr-Free Cobaltite-Based Perovskite Cathodes Directly Assembled on a Barrier-Layer-Free Y2O3-ZrO2 Electrolyte of Solid Oxide Fuel Cells. J. Mater. Chem. A 2017, 10, 993–1003. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, M.; Wang, N.; Ma, C.; Wang, J.; Han, M. A short review of cathode poisoning and corrosion in solid oxide fuel cell. Int. J. Hydrog. Energy 2017, 42, 24948–24959. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Ermakova, L.; Khrustov, A.; Farlenkov, A.; Bronin, D. Methods to increase electrochemical activity of lanthanum nickelate-ferrite electrodes for intermediate and low temperature SOFCs. Int. J. Hydrog. Energy 2021. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Shubin, K.; Ermakova, L.; Eremeev, N.; Farlenkov, A.; Khrustov, A.; Filonova, E.; Sadykov, V. Development of composite LaNi0·6Fe0.4O3–δ-based air electrodes for solid oxide fuel cells with a thin-film bilayer electrolyte. Int. J. Hydrog. Energy 2021, 46, 16947–16964. [Google Scholar] [CrossRef]
- Usuzaka, N.; Yamaguchi, H.; Watanabe, T. Preparation and magnetic properties of Co–Gd amorphous alloy films by the electroplating method. Mater. Sci. Eng. 1988, 99, 105–108. [Google Scholar] [CrossRef]
- Yu, C.; Kim, S.; Baek, J.D.; Li, Y.; Su, P.C.; Kim, T.S. Direct Observation of Nanoscale Pt Electrode Agglomeration at the Triple Phase Boundary. ACS Appl. Mater. Interfaces 2015, 7, 6036–6040. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Ji, S.; Park, J.; Lee, M.H.; Cha, S.W. Fuel Cells: Ultrathin YSZ Coating on Pt Cathode for High Thermal Stability and Enhanced Oxygen Reduction Reaction Activity. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Buyukaksoy, A.; Petrovsky, V.; Dogan, F. Solid Oxide Fuel Cells with Symmetrical Pt-YSZ Electrodes Prepared by YSZ Infiltration. J. Electrochem. Soc. 2013, 160, F482–F486. [Google Scholar] [CrossRef]
- Lee, S.; Seo, J.; Jung, W. Sintering-resistant Pt-CeO2 nanoparticles for high-temperature oxidation catalysis. Nanoscale 2016, 8, 10219–10228. [Google Scholar] [CrossRef] [Green Version]
- Ai, N.; Chen, K.; Jiang, S.P. A fundamental study of infiltrated CeO2 and (Gd,Ce)O2 nanoparticles on the electrocatalytic activity of Pt cathodes of solid oxide fuel cells. Solid State Ion. 2013, 233, 87–94. [Google Scholar] [CrossRef]
- Seo, H.G.; Choi, Y.; Koo, B.; Jang, A.; Jung, W. Robust nano-architectured composite thin films for a low-temperature solid oxide fuel cell cathode. J. Mater. Chem. A 2016, 4, 9394–9402. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.R.; Neoh, K.C.; Choi, H.J.; Han, G.D.; Jang, D.Y.; Kim, D.; Shim, J.H. Nanoporous silver cathode surface-treated by aerosol-assisted chemical vapor deposition of gadolinia-doped ceria for intermediate-temperature solid oxide fuel cells. J. Power Sources 2018, 402, 246–251. [Google Scholar] [CrossRef]
- Neoh, K.C.; Han, G.D.; Kim, M.; Kim, J.W.; Choi, H.J.; Park, S.W.; Shim, J.H. Nanoporous silver cathode surface treated by atomic layer deposition of CeOx for low-temperature solid oxide fuel cells. Nanotechnology 2016, 27, 185403. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, M.; Neoh, K.C.; Jang, D.Y.; Kim, H.J.; Shin, J.M.; Kim, G.T.; Shim, J.H. High-Performance Silver Cathode Surface Treated with Scandia-Stabilized Zirconia Nanoparticles for Intermediate Temperature Solid Oxide Fuel Cells. Adv. Energy Mater. 2016, 7, 1601956. [Google Scholar] [CrossRef]
- Lee, T.H.; Baek, J.D.; Fan, L.; Wiria, F.E.; Su, P.C.; Lee, S.H. SDC-Infiltrated Microporous Silver Membrane with Superior Resistance to Thermal Agglomeration for Cathode-Supported Solid Oxide Fuel Cells. Energies 2018, 11, 2181. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Seo, H.G.; Choi, Y.; Lim, D.K.; Jung, W. Cathodic electrochemical deposition: A new strategy to enhance the activity and stability of silver cathodes for thin-film solid oxide fuel cells. J. Mater. Chem. A 2020, 8, 14491–14497. [Google Scholar] [CrossRef]
- Chen, D.; Su, S.; Yu, Z.; Lu, L. Geometrical Optimization of the Composite Cathode in a Solid Oxide Fuel Cell. In Proceedings of the 2011 Asia-Pacific Power and Energy Engineering Conference, Wuhan, China, 25–28 March 2011; pp. 1–4. [Google Scholar] [CrossRef]
- Santillán, M.J.; Caneiro, A.; Quaranta, N.; Boccaccini, A.R. Electrophoretic deposition of La0.6Sr0.4Co0.8Fe0.2O3−δ cathodes on Ce0.9Gd0.1O1.95 substrates for intermediate temperature solid oxide fuel cell (IT-SOFC). J. Eur. Ceram. Soc. 2009, 29, 1125–1132. [Google Scholar] [CrossRef]
- Itagaki, Y.; Yahiro, H. Electrophoretic Deposition of Electrode Membrane for Solid Oxide Fuel Cells. ECS Meet. Abstr. 2018, MA2018-01, 1201. [Google Scholar] [CrossRef]
- Matsuda, M.; Hashimoto, M.; Matsunaga, C.; Suzuki, T.S.; Sakka, Y.; Uchikoshi, T. Electrophoretic fabrication of a-b plane oriented La2NiO4 cathode onto electrolyte in strong magnetic field for low-temperature operating solid oxide fuel cell. J. Eur. Ceram. Soc. 2016, 36, 4077–4082. [Google Scholar] [CrossRef]
- Santillán, M.J.; Caneiro, A.; Lovey, F.C.; Quaranta, N.; Boccaccini, A.R. Electrophoretic Codeposition of La0.6Sr0.4Co0.8Fe0.2O3−δ and Carbon Nanotubes for Developing Composite Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. Int. J. Appl. Ceram. Technol. 2010, 7, 30–40. [Google Scholar] [CrossRef]
- Asamoto, M.; Miyake, S.; Yonei, Y.; Yamaura, H.; Yahiro, H. Electrochemical Performances of Proton-Conducting SOFC with La-Sr-Fe-O Cathode Fabricated by Electrophoretic Deposition Techniques. Electrochemistry 2009, 77, 142–145. [Google Scholar] [CrossRef] [Green Version]
- Baque, L.C.; Soldati, A.; Troiani, H.; Schreiber, A.; Caneiro, A.; Serquis, A. Enhanced Oxygen Reduction Reaction Kinetics In Nanocrystalline IT-SOFC Cathodes. ECS Trans. 2013, 57, 2147–2156. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Muchtar, A.; Somalu, M.R.; Ali, S.A.M.; Rahman, H.A. Influence of sintering temperature on the polarization resistance of La0.6Sr0.4Co0.2Fe0.8O3–δ—SDC carbonate composite cathode. Ceram. Silikáty 2016, 60, 115–121. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, Z.; Mori, T.; Jiang, S.P. Development of nickel based cermet anode materials in solid oxide fuel cells – Now and future. Mater. Rep. Energy 2021, 1, 100003. [Google Scholar] [CrossRef]
- Medvedev, D.; Lyagaeva, J.; Vdovin, G.; Beresnev, S.; Demin, A.; Tsiakaras, P. A tape calendering method as an effective way for the preparation of proton ceramic fuel cells with enhanced performance. Electrochim. Acta 2016, 210, 681–688. [Google Scholar] [CrossRef]
- Agarkova, E.A.; Burmistrov, I.N.; Agarkov, D.A.; Zadorozhnaya, O.Y.; Shipilova, A.V.; Solovyev, A.A.; Nepochatov, Y.K.; Bredikhin, S.I. Bilayered anode supports for planar solid oxide fuel cells: Fabrication and electrochemical performance. Mater. Lett. 2021, 283, 128752–128757. [Google Scholar] [CrossRef]
- Besra, L.; Zha, S.; Liu, M. Preparation of NiO-YSZ/YSZ bi-layers for solid oxide fuel cells by electrophoretic deposition. J. Power Sources 2006, 160, 207–214. [Google Scholar] [CrossRef]
- Cherng, J.S.; Ho, M.Y.; Yeh, T.H.; Chen, W.H. Anode-supported micro-tubular SOFCs made by aqueous electrophoretic deposition. Ceram. Int. 2012, 38, S477–S480. [Google Scholar] [CrossRef]
- Itagaki, Y.; Shinohara, K.; Yamaguchi, S.; Yahiro, H. Anodic performance of bilayer Ni-YSZ SOFC anodes formed by electrophoretic deposition. J. Ceram. Soc. Jpn. 2015, 123, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Lomberg, M.; Ruiz-Trejo, E.; Offer, G.; Brandon, N.P. Characterization of Ni-Infiltrated GDC Electrodes for Solid Oxide Cell Applications. J. Electrochem. Soc. 2014, 161, F899–F905. [Google Scholar] [CrossRef]
- Klemensø, T.; Thydén, K.; Chen, M.; Wang, H.J. Stability of Ni–yttria stabilized zirconia anodes based on Ni-impregnation. J. Power Sources 2010, 195, 7295–7301. [Google Scholar] [CrossRef]
- Park, E.W.; Moon, H.; Park, M.; Hyun, S.H. Fabrication and characterization of Cu–Ni–YSZ SOFC anodes for direct use of methane via Cu-electroplating. Int. J. Hydrog. Energy 2009, 34, 5537–5545. [Google Scholar] [CrossRef]
- Gross, M.D.; Vohs, J.M.; Gorte, R.J. A study of thermal stability and methane tolerance of Cu-based SOFC anodes with electrodeposited Co. Electrochim. Acta 2007, 52, 1951–1957. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Gross, M.D.; Gorte, R.J.; Vohs, J.M. Electrodeposition of Cu into a Highly Porous Ni/YSZ Cermet. J. Electrochem. Soc. 2006, 153, A1539–A1543. [Google Scholar] [CrossRef] [Green Version]
- Gross, M.D.; Vohs, J.M.; Gorte, R.J. Enhanced Thermal Stability of Cu-Based SOFC Anodes by Electrodeposition of Cr. J. Electrochem. Soc. 2006, 153, A1386–A1390. [Google Scholar] [CrossRef]
- Jung, S.W.; Vohs, J.M.; Gorte, R.J. Preparation of SOFC Anodes by Electrodeposition. J. Electrochem. Soc. 2007, 154, B1270–B1275. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Trejo, E.; Atkinson, A.; Brandon, N.P. Metallizing porous scaffolds as an alternative fabrication method for solid oxide fuel cell anodes. J. Power Sources 2015, 280, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Jamil, Z.; Ruiz-Trejo, E.; Boldrin, P.; Brandon, N.P. Anode fabrication for solid oxide fuel cells: Electroless and electrodeposition of nickel and silver into doped ceria scaffolds. Int. J. Hydrog. Energy 2016, 41, 9627–9637. [Google Scholar] [CrossRef] [Green Version]
- Jamil, Z.; Ruiz-Trejo, E.; Brandon, N.P. Nickel Electrodeposition on Silver for the Development of Solid Oxide Fuel Cell Anodes and Catalytic Membranes. J. Electrochem. Soc. 2017, 164, D210–D217. [Google Scholar] [CrossRef]
- Ma, M.; Yang, X.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Progress and challenges of carbon-fueled solid oxide fuel cells anode. J. Energy Chem. 2021, 56, 209–222. [Google Scholar] [CrossRef]
- Smorygo, O.; Mikutski, V.; Marukovich, A.; Vialiuha, Y.; Ilyushchanka, A.; Mezentseva, N.; Alikina, G.; Vostrikov, Z.; Fedorova, Y.; Pelipenko, V.; et al. Structured catalyst supports and catalysts for the methane indirect internal steam reforming in the intermediate temperature SOFC. Int. J. Hydrog. Energy 2009, 34, 9505–9514. [Google Scholar] [CrossRef]
- Smirnova, A.; Sadykov, V.; Mezentseva, N.; Usoltsev, V.; Smorygo, O.; Bobrenok, O.; Uvarov, N. Metal-Supported SOFC on Compressed Ni-Al Foam Substrates. In Proceedings of the ASME 2010 8th International Fuel Cell Science, Engineering and Technology Conference, Brooklyn, NY, USA, 14–16 June 2010; pp. 411–416. [Google Scholar] [CrossRef]
- Melnik, J.; Fu, X.Z.; Luo, J.L.; Sanger, A.R.; Chuang, K.T.; Yang, Q.M. Ceria and copper/ceria functional coatings for electrochemical applications: Materials preparation and characterization. J. Power Sources 2010, 195, 2189–2195. [Google Scholar] [CrossRef]
- Fu, X.Z.; Melnik, J.; Low, Q.X.; Luo, J.L.; Chuang, K.T.; Sanger, A.R.; Yang, Q.M. Surface modified Ni foam as current collector for syngas solid oxide fuel cells with perovskite anode catalyst. Int. J. Hydrog. Energy 2010, 35, 11180–11187. [Google Scholar] [CrossRef]
- Low, Q.X.; Huang, W.; Fu, X.Z.; Melnik, J.; Luo, J.L.; Chuang, K.T.; Sanger, A.R. Copper coated nickel foam as current collector for H2S-containing syngas solid oxide fuel cells. Appl. Surf. Sci. 2011, 258, 1014–1020. [Google Scholar] [CrossRef]
- Lotfi, N.; Shahrabi, T.; Yaghoubinezhad, Y.; Darband, G.B. Direct electrodeposition of platinum nanoparticles@graphene oxide@nickel-copper@nickel foam electrode as a durable and cost-effective catalyst with remarkable performance for electrochemical hydrogen evolution reaction. Appl. Surf. Sci. 2020, 505, 144571. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; He, H.; Zhang, P. Electrodeposition of self-supported Ni–Fe–Sn film on Ni foam: An efficient electrocatalyst for oxygen evolution reaction. Electrochim. Acta 2019, 301, 39–46. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Y.; Zhang, Z.; Yang, L.; Zang, Q. Rapid electrodeposited of self-supporting Ni-Fe-Mo film on Ni foam as affordable electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2021, 390, 138754. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, X.; Liu, Y.; Pan, K.; Pang, H.; Wei, S. The application of CeO2-based materials in electrocatalysis. J. Mater. Chem. A 2019, 7, 17675–17702. [Google Scholar] [CrossRef]
- Yang, L.; Pang, X.; Fox-Rabinovich, G.; Veldhuis, S.; Zhitomirsky, I. Electrodeposition of cerium oxide films and composites. Surf. Coat. Technol. 2011, 206, 1–7. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Menshikova, A.V.; Nikolaenko, I.V. Electrophoretic deposition of a self-stabilizing suspension based on a nanosized multi-component electrolyte powder prepared by the laser evaporation method. Solid State Ion. 2016, 288, 110–114. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Kolchugin, A.A.; Pikalov, S.M.; Kaigorodov, A.S. Cyclic electrophoretic deposition of electrolyte thin-films on the porous cathode substrate utilizing stable suspensions of nanopowders. Solid State Ion. 2017, 302, 126–132. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Li, W.; Zhang, N.; Qi, H.; Finklea, H.; Liu, X. A study on the electrophoretic deposition of gadolinium doped ceria on polypyrrole coated yttrium stabilized zirconia. J. Colloid Interface Sci. 2019, 555, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Eba, H.; Anzai, C.; Ootsuka, S. Observation of Cation Diffusion and Phase Formation between Solid Oxide Layers of Lanthanum Gallate-Based Fuel Cells. Mater. Trans. 2018, 59, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Aldykiewicz, J.A.J.; Davenport, A.J.; Isaacs, H.S. Studies of the Formation of Cerium-Rich Protective Films Using X-ray Absorption Near-Edge Spectroscopy and Rotating Disk Electrode Methods. J. Electrochem. Soc. 1996, 143, 147–154. [Google Scholar] [CrossRef]
- Aldykiewicz, A.J.; Davenport, A.J.; Isaacs, H.S. The Effect of Borate on Cerium Film Formation. ECS Trans. 2007, 3, 143–151. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Petric, A. Electrolytic and electrophoretic deposition of CeO2 films. Mater. Lett. 1999, 40, 263–268. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Petric, A. Electrochemical deposition of ceria and doped ceria films. Ceram. Int. 2001, 27, 149–155. [Google Scholar] [CrossRef]
- Creus, J.; Brezault, F.; Rebere, C.; Gadouleau, M. Synthesis and characterisation of thin cerium oxide coatings elaborated by cathodic electrolytic deposition on steel substrate. Surf. Coat. Technol. 2006, 200, 4636–4645. [Google Scholar] [CrossRef]
- Li, F.B.; Newman, R.C.; Thompson, G.E. In situ atomic force microscopy studies of electrodeposition mechanism of cerium oxide films: Nucleation and growth out of a gel mass precursor. Electrochim. Acta 1997, 42, 2455–2464. [Google Scholar] [CrossRef]
- Dronova, M.; Lair, V.; Vermaut, P.; Ringuedé, A.; An, V. Study of ceria thin films prepared via electrochemical deposition: Role of selected electrochemical parameters on growth kinetics. Thin Solid Film. 2020, 693, 137674. [Google Scholar] [CrossRef]
- Valov, I.; Guergova, D.; Stoychev, D. A Study of the Kinetics of the Electrochemical Deposition of Ce3+/Ce4+ Oxides. In NATO Science for Peace and Security Series B: Physics and Biophysics; Springer: Dordrecht, The Netherlands, 2011; pp. 167–172. [Google Scholar] [CrossRef]
- Lair, V.; Żivković, L.; Lupan, O.; Ringuedé, A. Synthesis and characterization of electrodeposited samaria and samaria-doped ceria thin films. Electrochim. Acta 2011, 56, 4638–4644. [Google Scholar] [CrossRef]
- Żivković, L.; Lair, V.; Lupan, O.; Ringuedé, A. Effect of samarium addition and annealing on the properties of electrodeposited ceria thin films. Thin Solid Film. 2011, 519, 3538–3543. [Google Scholar] [CrossRef]
- Khalipova, O.S.; Lair, V.; Ringuedé, A. Electrochemical synthesis and characterization of Gadolinia-Doped Ceria thin films. Electrochim. Acta 2014, 116, 183–187. [Google Scholar] [CrossRef]
- Čerović, L.; Lair, V.; Lupan, O.; Cassir, M.; Ringuedé, A. Electrochemical synthesis and characterization of nanorods, nanocolumnar ceria—Based thin films on different glass substrates. Chem. Phys. Lett. 2010, 494, 237–242. [Google Scholar] [CrossRef]
- Phok, S.; Bhattacharya, R.N. Effect of samarium doping on electrodeposited CeO2 thin film. Phys. Status Solidi 2006, 203, 3734–3742. [Google Scholar] [CrossRef]
- Golden, T.D.; Wang, A.Q. Anodic Electrodeposition of Cerium Oxide Thin Films II. Mechanism Studies. J. Electrochem. Soc. 2003, 150, C621–C624. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Balasubramanian, M.; Melendres, C.; Mansour, A. An X-ray absorption study of the local structure of cerium in electrochemically deposited thin films. Thin Solid Film. 1999, 347, 178–183. [Google Scholar] [CrossRef]
- Wang, A.Q.; Golden, T.D. Anodic Electrodeposition of Cerium Oxide Thin Films: I. Formation of Crystalline Thin Films. J. Electrochem. Soc. 2003, 150, C616–C620. [Google Scholar] [CrossRef]
- Wang, A.Q.; Golden, T.D. Electrodeposition of Oriented Cerium Oxide Films. Int. J. Electrochem. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kulp, E.A.; Limmer, S.J.; Bohannan, E.W.; Switzer, J.A. Electrodeposition of nanometer-thick ceria films by oxidation of cerium(III)-acetate. Solid State Ion. 2007, 178, 749–757. [Google Scholar] [CrossRef]
- Azenha, M.E.; Burrows, H.D.; Fonseca, S.M.; Ramos, M.L.; Rovisco, J.; Seixas de Melo, J.; Sobral, A.J.F.N.; Kogej, K. Luminescence from cerium(III) acetate complexes in aqueous solution: Considerations on the nature of carboxylate binding to trivalent lanthanides. New J. Chem. 2008, 32, 1531–1535. [Google Scholar] [CrossRef]
- Kamada, K.; Enomoto, N.; Hojo, J. Optimization of electrochemical synthesis conditions for dense and doped ceria thin films. Electrochim. Acta 2009, 54, 6996–7000. [Google Scholar] [CrossRef] [Green Version]
- Lützke, F.; Maier, M.; Urbanska, A.; Zehbe, R.; Fleck, C.; Müller, W.D.; Mochales, C. Electrophoretic Deposition of Zirconia Multilayered Constructs. Key Eng. Mater. 2014, 631, 13–17. [Google Scholar] [CrossRef]
- Salehzadeh, D.; Torabi, M.; Sadeghian, Z.; Marashi, P. A multiscale-architecture solid oxide fuel cell fabricated by electrophoretic deposition technique. J. Alloy. Compd. 2020, 830, 154654–154664. [Google Scholar] [CrossRef]
- Ichiboshi, H.; Myoujin, K.; Kodera, T.; Ogihara, T. Preparation of Ce0.8Sm0.2O1.9 Thin Films by Electrophoretic Deposition and their Fuel Cell Performance. Key Eng. Mater. 2013, 566, 137–140. [Google Scholar] [CrossRef]
- Nakayama, S.; Miyayama, M. Fabrication and Fuel-Cell Properties of Sm-Doped CeO2 Electrolyte Film by Electrophoretic Deposition. Key Eng. Mater. 2007, 350, 175–178. [Google Scholar] [CrossRef]
- Yu, S.-M.; Lee, K.-T. Fabrication of GDC-based micro tubular SOFC single cell using electrophoretic deposition process. J. Ceram. Process. Res. 2016, 17, 290–294. [Google Scholar]
- Matsuda, M.; Hosomi, T.; Murata, K.; Fukui, T.; Miyake, M. Fabrication of bilayered YSZ/SDC electrolyte film by electrophoretic deposition for reduced-temperature operating anode-supported SOFC. J. Power Sources 2007, 165, 102–107. [Google Scholar] [CrossRef]
- Suzuki, H.T.; Uchikoshi, T.; Kobayashi, K.; Suzuki, T.S.; Sugiyama, T.; Furuya, K.; Matsuda, M.; Sakka, Y. Fabrication of GDC/LSGM/GDC tri-layers on polypyrrole-coated NiO-YSZ by electrophoretic deposition for anode-supported SOFC. J. Ceram. Soc. Jpn. 2009, 117, 1246–1248. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.T.; Uchikoshi, T.; Kobayashi, K.; Furuya, K.; Suzuki, T.S.; Sakka, Y.; Munakata, F. Fabrication of Ceria- and Lanthanium Gallate-based Solid Electrolyte Layers on Porous NiO-YSZ by Sequential Electrophoretic Deposition Process. J. Jpn. Soc. Powder Powder Metall. 2012, 59, 626–630. [Google Scholar] [CrossRef]
- Ishii, K.; Matsunaga, C.; Munakata, F.; Uchikoshi, T. Effect of A-site ion non-stoichiometry on the chemical stability and electric conductivity of strontium and magnesium-doped lanthanum gallate. J. Am. Ceram. Soc. 2019, 103, 790–799. [Google Scholar] [CrossRef]
- Seok, C.; Moon, J.; Park, M.; Hong, J.; Kim, H.; Son, J.; Lee, J.; Kim, B.; Lee, H.W.; Yoon, K.J. Low-temperature co-sintering technique for the fabrication of multi-layer functional ceramics for solid oxide fuel cells. J. Eur. Ceram. Soc. 2016, 36, 1417–1425. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Yao, M.; Li, T.; Liu, X. Electrophoretic Deposition of Gadolinium-doped Ceria as a Barrier Layer on Yttrium-stabilized Zirconia Electrolyte for Solid Oxide Fuel Cells. Fuel Cells 2017, 17, 869–874. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y. Modifying Suspensions for the Electrophoretic Deposition of BaCe0.5Zr0.3Y0.1Yb0.1O3–δ Solid Electrolyte. Russ. J. Phys. Chem. A. 2021, 95, 1934–1939. [Google Scholar] [CrossRef]
- Hu, S.; Finklea, H.; Li, W.; Li, W.; Qi, H.; Zhang, N.; Liu, X. Alternating Current Electrophoretic Deposition of Gadolinium Doped Ceria onto Yttrium Stabilized Zirconia: A Study of the Mechanism. ACS Appl. Mater. Interfaces 2020, 2, 11126–11134. [Google Scholar] [CrossRef]
- Kalinina, E.; Pikalova, E.; Ermakova, L.; Bogdanovich, N. Challenges of Formation of Thin-Film Solid Electrolyte Layers on Non-Conductive Substrates by Electrophoretic Deposition. Coatings 2021, 11, 805. [Google Scholar] [CrossRef]
- Safronov, A.P.; Kalinina, E.G.; Smirnova, T.A.; Leiman, D.V.; Bagazeev, A.V. Self-stabilization of aqueous suspensions of alumina nanoparticles obtained by electrical explosion. Russ. J. Phys. Chem. A. 2010, 84, 2122–2127. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Bogdanovich, N.M.; Bronin, D.I.; Pikalova, E.Y.; Pankratov, A.A. Formation of Thin-Film Electrolyte by Electrophoretic Deposition onto Modified Multilayer Cathode. Russ. J. Appl. Chem. 2019, 92, 191–198. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sato, K.; Matsuda, M.; Ozawa, M.; Ohara, S. Anomalous low-temperature sintering of a solid electrolyte thin film of tailor-made nanocrystals on a porous cathode support for low-temperature solid oxide fuel cells. Ceram. Int. 2021, 47, 15939–15946. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hashishin, T.; Matsuda, M.; Qiu, N.; Tan, Z.; Ohara, S. High-performance Ni nanocomposite anode fabricated from Gd-doped ceria nanocubes for low-temperature solid-oxide fuel cells. Nano Energy 2014, 6, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Shu, L.; Sunarso, J.; Hashim, S.S.; Mao, J.; Zhou, W.; Liang, F. Advanced perovskite anodes for solid oxide fuel cells: A review. Int. J. Hydrog. Energy 2019, 44, 31275–31304. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.J.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling—A critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Steward, D.; Mayyas, A.; Mann, M. Economics and Challenges of Li-Ion Battery Recycling from End-of-Life Vehicles. Procedia Manuf. 2019, 33, 272–279. [Google Scholar] [CrossRef]
- Abbas, Q.; Mirzaeian, M.; Hunt, M.R.C.; Hall, P.; Raza, R. Current State and Future Prospects for Electrochemical Energy Storage and Conversion Systems. Energies 2020, 13, 5847. [Google Scholar] [CrossRef]
- Peksen, M. Hydrogen Technology to-wards the Solution of Environment-Friendly New Energy Vehicles. Energies 2021, 14, 4892. [Google Scholar] [CrossRef]
- Mehmeti, A.; McPhail, S.J.; Pumiglia, D.; Carlini, M. Life cycle sustainability of solid oxide fuel cells: From methodo-logical aspects to system implications. J. Power Sources 2016, 235, 772–785. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.; Miller, E.C.; Railsback, J.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Mulone, A.; Hildenbrand, J.; Klement, U. Electrodeposition: Three steps towards sustainability. Trans. Inst. Met. Finish. 2020, 98, 108–113. [Google Scholar] [CrossRef]















| Cell Configuration | Power Density, W/cm2 (T, °C) | ASR, Ω·cm2 (T, °C) | Long-Term Study | Ref. |
|---|---|---|---|---|
| NiO-YSZ/NiO-ScCeSZ/ScCeSZ/LCO 5-ScCeSZ (nanofibrous LCO-GDC prepared by CAED) NiO-YSZ/NiO-ScCeSZ/ScCeSZ/LCO-ScCeSZ (screen-printed LCO-ScCeSZ) | 0.591 (700) 0.781 (750) 0.956 (800) 0.360 (800) | 1.25 (700) 0.93 (750) 0.71 (800) 1.17 (800) | Increase in the cell voltage during the first 20 h from 0.61 to 0.65 V at 1 A/cm2 (750 °C). 200 h cell testing, no degradation. | [52] |
| NiO-YSZ 1/NiO-ScCeSZ 2/ScCeSZ/LNO 3-GDC 4 (LNO-GDC modification by CAED) NiO-YSZ/NiO-ScCeSZ/ScCeSZ/LNO-GDC (LNO-GDC prepared by sintering, without modification) | 0.477 (650) 0.717 (700) 0.974 (750) 0.528 (750) | 1.632 (650) 0.985 (700) 0.625 (750) 2.557 (650) 1.515 (700) 0.884 (750) | Sharp increase in the cell voltage during the first 24 h. 100 h cell testing at 1 A/cm2 (750 °C), no degradation. | [53] |
| NiO-YSZ/NiO-ScCeSZ/ScCeSZ/LNF 6-GDC (nanofibrous LNF-GDC prepared using hybrid ELD (electroplating + CAED) NiO-YSZ/NiO-ScCeSZ/ScCeSZ/LNF-GDC NiO-YSZ/NiO-ScCeSZ/ScCeSZ/LSCF 7-GDC (screen-printed LNF-GDC and LSCF-GDC) | 0.61 (700) 0.98 (750) 1.32 (800) 0.476 (750) 0.874 (750) | 0.415 (700) 0.284 (750) 0.223 (800) 0.898 (750) 0.379 (750) | At 0.64 A/cm2 (750 °C), Cr-poisoning conditions: 300 h, no degradation. Drop in the cell voltage: 100 h, 6% (LNF-GDC) 100 h, 9.6% (LSCF-GDC) | [54] |
| Pt-SDC 8/YSZ/Pt-SDC (Pt electrode CELD-covered with SDC) Pt/YSZ/Pt (conventional sputter-deposited Pt electrode) | 44 (450) 224 (400) 4810 (450) 38300 (400) | 100 h testing at 600 °C, no degradation. 40 h testing, 146% degradation. | [66] | |
| Ag-PCO 9/YSZ/Ag-PCO (Ag electrode CELD-covered with PCO) Ag/YSZ/Ag (conventional sputter-deposited Ag electrode) | 0.067 (450) 0.048 (450) | 3.7 (450) 121 (450) | 100 h testing at 550 °C, no degradation. 70 h testing at 450 °C, initial degradation before stabilization (20 times for 10 min) | [71] |
| NiO-GDC/GDC/LN 10 Oriented LN deposited by EPD in magnetic field NiO-GDC/GDC/LN Chaotically oriented LN | 0.011 (500) 0.008 (500) | 12 (500) 25 (500) | [75] | |
| Pd-CeO2-YSZ/YSZ/LSM 11-YSZ LSM-YSZ obtained by EPD Pd-CeO2-YSZ/YSZ/LSM-YSZ LSM infiltrated into YSZ backbone | Ohmic losses 0.65 and 0.55 Ω cm2 (before and after shorting). Ohmic losses 0.45 Ω cm2 (before and after shorting) (700 °C) | [49] | ||
| Cu-Ni-YSZ/YSZ/LSM-YSZ Cu electroplating Ni-YSZ anode Ni-YSZ/YSZ/LSM-YSZ without modification | 0.32 (700) H2 0.24 (700) CH4 0.38 (700) H2 0.28 (700) CH4 | 1.68 (700) H2 2.18 (700) CH4 1.00 (700) H2 1.96 (700) CH4 | 0.17 W/cm2 and 2.75 Ω·cm2 after 200 h testing using CH4 (700 °C) Stepwise degradation and cracking after 21 h | [88] |
| Co-Cu–ceria–YSZ/YSZ/LSM-YSZ 13 vol% Cu, Co modification by ED (5 vol%) Co-Cu–ceria–YSZ/YSZ/LSM-YSZ 13 vol% Cu, Co impregnated (5 vol%) Cu–ceria–YSZ/YSZ/LSM-YSZ 18 vol% Cu, without modification | 0.375 (900) 0.120 (900) | 0.80 (900) 0.88 (900) | Before/after 50 h testing at 900 °C: Ohmic losses 0.50/0.72 Ω cm2 Ohmic losses 0.80/2.00 Ω cm2 Ohmic losses 0.88/2.40 Ω cm2 | [89] |
| Ni-GDC/YSZ/Ni-GDC Ni electrodeposited on GDC backbone Ni-GDC/YSZ/Ni-GDC Ni infiltrated GDC | 1.00 (700) wet H2 0.168 (700) wet H2 | [86,93] | ||
| Ni-Ag-GDC/YSZ/GDC-Ag-Ni Ni electrodeposited on Ag-GDC backbone Ag-GDC/YSZ/GDC-Ag | 1.12 (750) wet H2 3.77 (750) H2 | [94] | ||
| Cu-Ni-foam-LSCM 12-YSZ-Pt Ni-foam collector Cu-modified by plating Au-LSCM-YSZ-Pt | 0.155 (900) Syngas13 0.140 (900) | 13.5 (900) syngas 15.0 (900) | 48 h testing using syngas as a fuel (900 °C), no degradation | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, E.; Pikalova, E. Opportunities, Challenges and Prospects for Electrodeposition of Thin-Film Functional Layers in Solid Oxide Fuel Cell Technology. Materials 2021, 14, 5584. https://doi.org/10.3390/ma14195584
Kalinina E, Pikalova E. Opportunities, Challenges and Prospects for Electrodeposition of Thin-Film Functional Layers in Solid Oxide Fuel Cell Technology. Materials. 2021; 14(19):5584. https://doi.org/10.3390/ma14195584
Chicago/Turabian StyleKalinina, Elena, and Elena Pikalova. 2021. "Opportunities, Challenges and Prospects for Electrodeposition of Thin-Film Functional Layers in Solid Oxide Fuel Cell Technology" Materials 14, no. 19: 5584. https://doi.org/10.3390/ma14195584
APA StyleKalinina, E., & Pikalova, E. (2021). Opportunities, Challenges and Prospects for Electrodeposition of Thin-Film Functional Layers in Solid Oxide Fuel Cell Technology. Materials, 14(19), 5584. https://doi.org/10.3390/ma14195584