Raphia-Microorganism Composite Biosorbent for Lead Ion Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
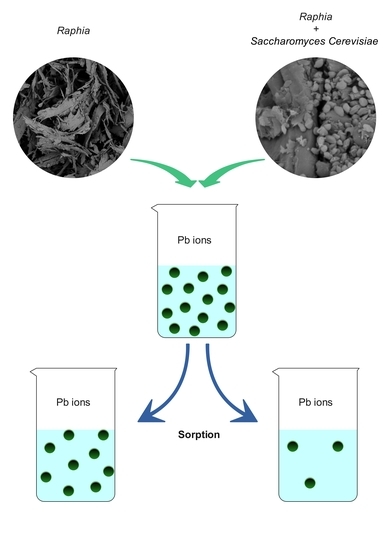
2.2. Preparation of RA-SC
2.3. Characterization
2.4. Sorption Process
2.4.1. Choosing the Appropriate Metal Ions
2.4.2. Specific Sorption Studies
2.5. Statistical Analysis of the Fitted Models
2.6. Equilibrium Studies
2.7. Kinetic Studies
3. Results and Discussion
3.1. Characteristic of RA and RA-SC
3.2. Effect of Time and Initial Concentration on Metal Ion Sorption
3.3. Equilibrium Studies
3.4. Kinetic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nat. Cell Biol. 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Baniamerian, M.J.; Moradi, S.E.; Noori, A.; Salahi, H. The effect of surface modification on heavy metal ion removal from water by carbon nanoporous adsorbent. Appl. Surf. Sci. 2009, 256, 1347–1354. [Google Scholar] [CrossRef]
- Mudhoo, A.; Garg, V.K.; Wang, S. Removal of heavy metals by biosorption. Environ. Chem. Lett. 2011, 10, 109–117. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Zhang, L.; Wang, M.; Zhao, M. Application of bifunctional Saccharomyces cerevisiae to remove lead(II) and cadmium(II) in aqueous solution. Appl. Surf. Sci. 2011, 257, 9809–9816. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Concha-Lozano, N.; Renard, F.; Quirico, E. Removal of oxyanions from synthetic wastewater via carbonation process of calcium hydroxide: Applied and fundamental aspects. J. Hazard. Mater. 2009, 166, 788–795. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xu, J.; Bu, X.H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents–A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tong, M.; Sun, X.; Li, B. Cystine-modified biomass for Cd(II) and Pb(II) biosorption. J. Hazard. Mater. 2007, 143, 277–284. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Martins, S.C.S.; Martins, C.M.; Fiúza, L.M.C.G.; Santaella, S.T. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol. 2016, 12, 4473. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; KumarChintalpudi, V.; Muddada, S. Application of Biosorption for Removal of Heavy Metals from Wastewater. Biosorption 2018, 69–116. [Google Scholar] [CrossRef] [Green Version]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Chwastowski, J.; Staroń, P. Influence of Saccharomyces cerevisiae yeast cells immobilized on Cocos nucifera fibers for the adsorption of Pb(II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 632, 127735. [Google Scholar] [CrossRef]
- Sarioglu, O.F.; Celebioglu, A.; Tekinay, T.; Uyar, T. Bacteria-immobilized electrospun fibrous polymeric webs for hexavalent chromium remediation in water. Int. J. Environ. Sci. Technol. 2016, 13, 2057–2066. [Google Scholar] [CrossRef]
- Khattar, J.I.S.; Sarma, T.A.; Singh, D.P. Removal of chromium ions by agar immobilized cells of the cyanobacterium Anacystis nidulans in a continuous flow bioreactor. Enzyme Microb. Technol. 1999, 25, 564–568. [Google Scholar] [CrossRef]
- Liu, J.; Morales-Narváez, E.; Vicent, T.; Merkoçi, A.; Zhong, G.H. Microorganism-decorated nanocellulose for efficient diuron removal. Chem. Eng. J. 2018, 354, 1083–1091. [Google Scholar] [CrossRef]
- Ahmad, A.; Singh, A.P.; Khan, N.; Chowdhary, P.; Giri, B.S.; Varjani, S.; Chaturvedi, P. Bio-composite of Fe-sludge biochar immobilized with Bacillus Sp. in packed column for bio-adsorption of Methylene blue in a hybrid treatment system: Isotherm and kinetic evaluation. Environ. Technol. Innov. 2021, 23, 101734. [Google Scholar] [CrossRef]
- Staroń, P.; Sorys, P.; Chwastowski, J. Equilibrium and Kinetic Study of Ammonium Sorption by Raphia farinifera. Water. Air. Soil Pollut. 2019, 230. [Google Scholar] [CrossRef] [Green Version]
- Staroń, P.; Chwastowski, J.; Banach, M. Sorption behavior of methylene blue from aqueous solution by raphia fibers. Int. J. Environ. Sci. Technol. 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Ofomaja, A.E.; Ho, Y.S. Effect of pH on cadmium biosorption by coconut copra meal. J. Hazard. Mater. 2007, 139, 356–362. [Google Scholar] [CrossRef]
- Marques, B.S.; Frantz, T.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A.; Dotto, G.L. Adsorption of a textile dye onto piaçava fibers: Kinetic, equilibrium, thermodynamics, and application in simulated effluents. Environ. Sci. Pollut. Res. 2019, 26, 28584–28592. [Google Scholar] [CrossRef]
- Marques, J.L.; Lütke, S.F.; Frantz, T.S.; Espinelli, J.B.S.; Carapelli, R.; Pinto, L.A.A.; Cadaval, T.R.S. Removal of Al (III) and Fe (III) from binary system and industrial effluent using chitosan films. Int. J. Biol. Macromol. 2018, 120, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef]
- El-Sayed, M.T. Removal of lead(II) by Saccharomyces cerevisiae AUMC 3875. Ann. Microbiol. 2013, 63, 1459–1470. [Google Scholar] [CrossRef]
- Say, R.; Denizli, A.; Yakup Arica, M. Biosorption of cadmium(II), lead(II) and copper(II) with the filamentous fungus Phanerochaete chrysosporium. Bioresour. Technol. 2001, 76, 67–70. [Google Scholar] [CrossRef]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and Equilibrium Models of Adsorption. Carbon Nanostructures 2015, 0, 33–69. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Baláž, P.; Aláčová, A.; Briančin, J. Sensitivity of Freundlich equation constant 1/n for zinc sorption on changes induced in calcite by mechanical activation. Chem. Eng. J. 2005, 114, 115–121. [Google Scholar] [CrossRef]
- Mousa, S.M.; Ammar, N.S.; Ibrahim, H.A. Removal of lead ions using hydroxyapatite nano-material prepared from phosphogypsum waste. J. Saudi Chem. Soc. 2016, 20, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Cheung, C.W.; Porter, J.F.; McKay, G. Sorption kinetics for the removal of copper and zinc from effluents using bone char. Sep. Purif. Technol. 2000, 19, 55–64. [Google Scholar] [CrossRef]
- Kumar, K.V. Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J. Hazard. Mater. 2006, 137, 1538–1544. [Google Scholar] [CrossRef]
- López-Luna, J.; Ramírez-Montes, L.E.; Martinez-Vargas, S.; Martínez, A.I.; Mijangos-Ricardez, O.F.; González-Chávez, M.D.C.A.; Carrillo-González, R.; Solís-Domínguez, F.A.; del Carmen Cuevas-Díaz, M.; Vázquez-Hipólito, V. Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl. Sci. 2019, 1, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Svilović, S.; Rušić, D.; Bašić, A. Investigations of different kinetic models of copper ions sorption on zeolite 13X. Desalination 2010, 259, 71–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, C.; Meng, Q.; Diao, Z.; Dong, L.; Peng, X.; Ma, S.; Zhou, Q. Biosorption of Pb2+ by Saccharomyces Cerevisiae in Static and Dynamic Adsorption Tests. Bull. Environ. Contam. Toxicol. 2009, 83, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Evora, M.C.; Gonçalez, O.L.; Dutra, R.C.L.; Diniz, M.F.; Wiebeck, H.; de Andrade e Silva, L.G. Comparação de Técnicas FTIR de Transmissão, Reflexão e Fotoacústica na Análise de Poliamida-6, Reciclada e Irradiada. Polímeros 2002, 12, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Cavagna, M.; Dell’Anna, R.; Monti, F.; Rossi, F.; Torriani, S. Use of ATR-FTIR Microspectroscopy to Monitor Autolysis of Saccharomyces cerevisiae Cells in a Base Wine. J. Agric. Food Chem. 2009, 58, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Mitter, E.K.; Corso, C.R. FT-IR analysis of acid black dye biodegradation using saccharomyces cerevisiae immobilized with treated sugarcane bagasse. Water. Air. Soil Pollut. 2013, 224, 1–9. [Google Scholar] [CrossRef]
- Wahab, M.A.; Boubakri, H.; Jellali, S.; Jedidi, N. Characterization of ammonium retention processes onto Cactus leaves fibers using FTIR, EDX and SEM analysis. J. Hazard. Mater. 2012, 241–242, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.; Rebufa, C.; Dupuy, N.; Kister, J. FTIR—Multivariate curve resolution monitoring of photo-Fenton degradation of phenolic aqueous solutions: Comparison with HPLC as a reference method. Talanta 2008, 77, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Cherdoud-Chihani, A.; Mouzali, M.; Abadie, M.J.M. Study of crosslinking acid copolymer/DGEBA systems by FTIR. J. Appl. Polym. Sci. 2003, 87, 2033–2051. [Google Scholar] [CrossRef]
- El-Kabbany, F.; Hassan, T.S.; Hafez, M. Infrared spectroscopic anaylsis of polymorphism in diphenyl carbazide. Sci. Pap. Univ. Pardubice. Ser. A Fac. Chem. Technol. 2010, 16, 57–73. [Google Scholar]
- Pistorius, A.M.A.; DeGrip, W.J.; Egorova-Zachernyuk, T.A. Monitoring of biomass composition from microbiological sources by means of FT-IR spectroscopy. Biotechnol. Bioeng. 2009, 103, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J. The characteristics and mechanism of Co(II) removal from aqueous solution by a novel xanthate-modified magnetic chitosan. Nucl. Eng. Des. 2012, 242, 452–457. [Google Scholar] [CrossRef]
- Sathya, D.J.H.; Turakhia, A.M.; Kumar, M.A.; Balaji, N.; Selvanaveen, S.; Vinodhini, G.; Seenuvasan, M. Bioethanol from saccharificed lignocellulosic rich Aloe vera rinds using Saccharomyces cerevisiae MTCC 4779. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1347–1352. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.K.; Renganayaki, V.; Natarajan, S. Vibrational spectra and thermodynamic analysis of metformin. Indian J. Pure Appl. Phys. 2006, 44, 495–500. [Google Scholar]
- Da Luz, A.D.; de Souza, S.M.D.A.G.U.; da Luz, C.; de Mello, J.M.M.; de Souza, A.A.U. Analysis of Competition between Multicomponent BTX Compounds for the Active Site of Adsorption in a Fixed-Bed Column. Ind. Eng. Chem. Res. 2013, 52, 16911–16921. [Google Scholar] [CrossRef]
- Al-Rub, F.A.A.; El-Naas, M.; Benyahia, F.; Ashour, I. Biosorption of nickel on blank alginate beads, free and immobilized algal cells. Process Biochem. 2004, 39, 1767–1773. [Google Scholar] [CrossRef]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef] [Green Version]
- Ngah, W.S.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, Z.; Ma, W. Adsorption of Pb(II) from glucose solution on thiol-functionalized cellulosic biomass. Bioresour. Technol. 2012, 104, 807–809. [Google Scholar] [CrossRef]
- Pereira, F.V.; Gurgel, L.V.A.; Gil, L.F. Removal of Zn2+ from aqueous single metal solutions and electroplating wastewater with wood sawdust and sugarcane bagasse modified with EDTA dianhydride (EDTAD). J. Hazard. Mater. 2010, 176, 856–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelnaeim, M.Y.; El Sherif, I.Y.; Attia, A.A.; Fathy, N.A.; El-Shahat, M.F. Impact of chemical activation on the adsorption performance of common reed towards Cu(II) and Cd(II). Int. J. Miner. Process. 2016, 157, 80–88. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Mu, T.-H.; Sun, H.-N. Sweet Potato Leaf Polyphenols: Preparation, Individual Phenolic Compound Composition and Antioxidant Activity. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: London, UK, 2019; pp. 365–380. [Google Scholar]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Özacar, M.; Şengil, I.A.; Türkmenler, H. Equilibrium and kinetic data, and adsorption mechanism for adsorption of lead onto valonia tannin resin. Chem. Eng. J. 2008, 143, 32–42. [Google Scholar] [CrossRef]
- Özacar, M.; Şengil, I.A. Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour. Technol. 2005, 96, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Mouni, L.; Merabet, D.; Bouzaza, A.; Belkhiri, L. Adsorption of Pb(II) from aqueous solutions using activated carbon developed from Apricot stone. Desalination 2011, 276, 148–153. [Google Scholar] [CrossRef]
- Akinhanmi, F.T.; Adeogun, A.I.; Adegbuyi, A. Removal of Cu2+ from aqueous solution by adsorption onto quail eggshell: Kinetic and isothermal studies. J. Environ. Biotechnol. Res. 2016, 5, 1–9. [Google Scholar]
- Khan, T.A.; Chaudhry, S.A.; Ali, I. Equilibrium uptake, isotherm and kinetic studies of Cd(II) adsorption onto iron oxide activated red mud from aqueous solution. J. Mol. Liq. 2015, 202, 165–175. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Puad, N.A.A.; Bello, O.S. Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resour. Ind. 2014, 6, 18–35. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Liu, S.; Zhang, B.; Xu, L.; Hu, X. Equilibrium and kinetics studies on the absorption of Cu(II) from the aqueous phase using a β-cyclodextrin-based adsorbent. Carbohydr. Polym. 2012, 88, 609–617. [Google Scholar] [CrossRef]
- Özacar, M.; Şengil, I.A. A kinetic study of metal complex dye sorption onto pine sawdust. Process Biochem. 2005, 40, 565–572. [Google Scholar] [CrossRef]
- Örnek, A.; Özacar, M.; Şengil, I.A. Adsorption of lead onto formaldehyde or sulphuric acid treated acorn waste: Equilibrium and kinetic studies. Biochem. Eng. J. 2007, 37, 192–200. [Google Scholar] [CrossRef]
- Riahi, K.; Chaabane, S.; Thayer, B. Ben A kinetic modeling study of phosphate adsorption onto Phoenix dactylifera L. date palm fibers in batch mode. J. Saudi Chem. Soc. 2017, 21, S143–S152. [Google Scholar] [CrossRef] [Green Version]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef] [Green Version]





| Isotherm Models | Equation | Reference | No. Equation |
|---|---|---|---|
| Sorption capacity | [24] | (1) | |
| The determination coefficient | [25] | (2) | |
| The average relative error | [26] | (3) |
| Isotherm Models | Equation | Reference | No. Equation |
|---|---|---|---|
| Langmuir | [31] | (4) | |
| Freundlich | [32] | (5) | |
| Temkin | [33] | (6) | |
| Dubinin–Radushkevich | [34] | (7) | |
| (8) |
| Kinetic Models | Equation | Reference | No. Equation |
|---|---|---|---|
| Pseudo-first-order | [36] | (9) | |
| Pseudo-second-order | [37] | (10) | |
| Elovich | [38] | (11) | |
| Weber–Morris | [39] | (12) |
| Isotherm Model | Parameters | ||||
|---|---|---|---|---|---|
| Langmuir | ARE [%] | R2 | qm [mg/g] | KL [dm3/mg] | |
| RA | 3.18 | 0.9583 | 17.139 | 0.0155505 | |
| RA-SC | 2.82 | 0.9934 | 106.089 | 0.1985247 | |
| Freundlich | ARE [%] | R2 | KF (mg1−(1/n)(dm3)1/ng−1) | 1/n | |
| RA | 1.80 | 0.9839 | 2.81066 | 0.28086 | |
| RA-SC | 6.49 | 0.9705 | 30.61679 | 0.32560 | |
| Temkin | ARE [%] | R2 | KT [dm3/g] | B | |
| RA | 1.77 | 0.9884 | 0.2194991 | 3.347 | |
| RA-SC | 0.95 | 0.9984 | 2.2944000 | 21.711 | |
| D-R | ARE [%] | R2 | Kad [mol2/kJ2] | qd [mg/g] | |
| RA | 3.99 | 0.9353 | 0.0171906 | 16.199 | |
| RA-SC | 6.36 | 0.9683 | 0.0012690 | 98.249 | |
| Kinetic Model | Lead Ion Concentration C0 [mg/dm3] | |||||
|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | ||
| Pseudo-first-order rate | ||||||
| RA | q1 [mg/g] | 8.190 | 10.073 | 11.452 | 12.334 | 14.547 |
| k1 [min−1] | 1.5451 | 1.7929 | 1.8558 | 1.8258 | 1.6270 | |
| R2 | 0.7189 | 0.5612 | 0.6158 | 0.6137 | 0.6502 | |
| ARE [%] | 9.80 | 11.19 | 9.63 | 9.82 | 10.53 | |
| RA-SC | q1 [mg/g] | 31.328 | 51.796 | 65.565 | 76.454 | 81.915 |
| k1 [min−1] | 3.3515 | 1.2194 | 0.9935 | 0.7753 | 0.8181 | |
| R2 | 0.6748 | 0.8125 | 0.8051 | 0.7740 | 0.6878 | |
| ARE [%] | 3.70 | 9.02 | 10.50 | 13.25 | 14.94 | |
| Pseudo-second-order rate | ||||||
| RA | q2 [mg/g] | 8.777 | 10.789 | 12.197 | 13.153 | 15.618 |
| k2 [min−1] | 0.2534 | 0.2354 | 0.2258 | 0.2041 | 0.1465 | |
| R2 | 0.9088 | 0.7963 | 0.8387 | 0.8382 | 0.8623 | |
| ARE [%] | 5.56 | 7.65 | 6.21 | 6.43 | 6.57 | |
| RA-SC | q2 [mg/g] | 32.222 | 56.295 | 72.189 | 85.027 | 91.513 |
| k2 [min−1] | 0.2445 | 0.0290 | 0.0176 | 0.0117 | 0.0111 | |
| R2 | 0.9074 | 0.9554 | 0.9482 | 0.9270 | 0.8754 | |
| ARE [%] | 1.94 | 4.54 | 5.57 | 7.52 | 9.73 | |
| Elovich | ||||||
| RA | β [g/mg] | 0.9763 | 0.8080 | 0.7539 | 0.6908 | 0.5424 |
| α [mg/g·min] | 332.8 | 514.7 | 892.4 | 866.1 | 566.0 | |
| R2 | 0.9753 | 0.9830 | 0.9948 | 0.9925 | 0.9915 | |
| ARE [%] | 2.35 | 2.21 | 1.02 | 1.32 | 1.64 | |
| RA-SC | β [g/mg] | 0.5966 | 0.1325 | 0.0940 | 0.0735 | 0.0685 |
| α [mg/g·min] | 28,433,115 | 737.9 | 487.4 | 334.8 | 373.3 | |
| R2 | 0.9825 | 0.9818 | 0.9801 | 0.9825 | 0.9772 | |
| ARE [%] | 0.94 | 2.62 | 2.74 | 3.01 | 4.18 | |
| Weber-Morris | ||||||
| RA | I | 5.3411 | 6.6159 | 7.7652 | 8.3106 | 9.3924 |
| Kid | 0.8144 | 1.0401 | 1.1002 | 1.1985 | 1.5125 | |
| R2 | 0.8300 | 0.9353 | 0.9206 | 0.9153 | 0.8989 | |
| ARE [%] | 7.56 | 4.15 | 4.47 | 4.67 | 5.71 | |
| RA-SC | I | 26.9339 | 30.4228 | 34.8629 | 35.9809 | 38.5482 |
| Kid | 1.3367 | 5.9555 | 8.4153 | 10.8642 | 11.9007 | |
| R2 | 0.8393 | 0.8266 | 0.8358 | 0.8714 | 0.9086 | |
| ARE [%] | 2.57 | 9.45 | 10.60 | 10.14 | 7.79 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staroń, P.; Chwastowski, J. Raphia-Microorganism Composite Biosorbent for Lead Ion Removal from Aqueous Solutions. Materials 2021, 14, 7482. https://doi.org/10.3390/ma14237482
Staroń P, Chwastowski J. Raphia-Microorganism Composite Biosorbent for Lead Ion Removal from Aqueous Solutions. Materials. 2021; 14(23):7482. https://doi.org/10.3390/ma14237482
Chicago/Turabian StyleStaroń, Paweł, and Jarosław Chwastowski. 2021. "Raphia-Microorganism Composite Biosorbent for Lead Ion Removal from Aqueous Solutions" Materials 14, no. 23: 7482. https://doi.org/10.3390/ma14237482
APA StyleStaroń, P., & Chwastowski, J. (2021). Raphia-Microorganism Composite Biosorbent for Lead Ion Removal from Aqueous Solutions. Materials, 14(23), 7482. https://doi.org/10.3390/ma14237482