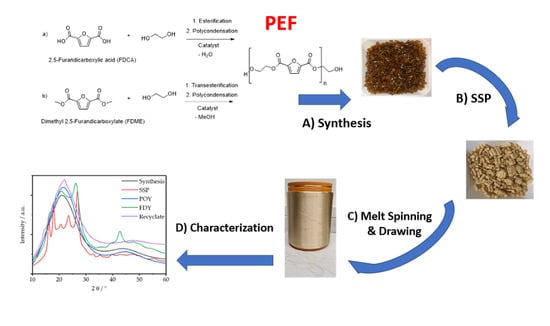
Poly(Ethylene Furanoate) along Its Life-Cycle from a Polycondensation Approach to High-Performance Yarn and Its Recyclate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. General Procedure for PEF Synthesis
2.3. Solid-State Polycondensation
2.4. Intrinsic Viscosity Measurements
2.5. Carboxyl End Group (CEG) Determination
2.6. Size Exclusion Chromatography (SEC)
2.7. Determination of the Moisture Content
2.8. Shear Rheological Characterization
2.9. Melt Spinning
2.10. Drawing of As-Spun Yarns
2.11. Testing of Yarn Titer
2.12. Tensile Testing
2.13. Differential Scanning Calorimetry (DSC)
2.14. X-ray Diffraction
2.15. Filament Recycling
3. Results and Discussion
3.1. PEF Synthesis
3.2. Spinning of PEF
3.3. Characterization of the Crystal Structure of PEF Along the Entire Yarn “Life-Cycle”
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Körner, P.; Beil, S.; Kruse, A. Effect of salt on the formation of 5-hydroxymethylfurfural from ketohexoses under aqueous conditions. React. Chem. Eng. 2019, 4, 747–762. [Google Scholar] [CrossRef]
- Körner, P.; Jung, D.; Kruse, A. Kinetic study on the impact of acidity and acid concentration on the formation of 5-hydroxymethylfurfural (HMF), humins, and levulinic acid in the hydrothermal conversion of fructose. Biomass Convers. Biorefin. 2019. [Google Scholar] [CrossRef]
- Stökle, K.; Hülsemann, B.; Steinbach, D.; Cao, Z.; Oechsner, H.; Kruse, A. A biorefinery concept using forced chicory roots for the production of biogas, hydrochar, and platform chemicals. Biomass Convers.Biorefin. 2019. [Google Scholar] [CrossRef]
- Stökle, K.; Kruse, A. Extraction of sugars from forced chicory roots. Biomass Convers.Biorefin. 2019, 9, 699–708. [Google Scholar] [CrossRef]
- Beer, B.; Pick, A.; Döring, M.; Lommes, P.; Sieber, V. Substrate scope of a dehydrogenase from Sphingomonas species A1 and its potential application in the synthesis of rare sugars and sugar derivatives. Microb. Biotechnol. 2018, 11, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of catalyst type on recyclability and decomposition mechanism of poly (ethylene furanoate) biobased polyester. J. Anal. Appl. Pyrolysis. 2017, 126, 357–370. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly (ethylene furanoate) compared to poly (ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Terzopoulou, Z.; Kyzas, G.; Bikiaris, D.; Lambropoulou, D. Biobased poly (ethylene furanoate) polyester/TiO2 supported nanocomposites as effective photocatalysts for anti-inflammatory/analgesic drugs. Molecules 2019, 24, 564. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, X.; Zhu, J.; Jiang, Y. Copolyesters based on 2,5-furandicarboxylic acid (FDCA): Effect of 2,2,4,4-tetramethyl-1,3-cyclobutanediol units on their properties. Polymers 2017, 9, 305. [Google Scholar] [CrossRef] [Green Version]
- Gandini, A.; Silvestre, A.J.D.; Neto, C.P.; Sousa, A.F.; Gomes, M. The furan counterpart of poly (ethylene terephthalate): An alternative material based on renewable resources. J. Polym. Sci. A Polym. Chem. 2009, 47, 295–298. [Google Scholar] [CrossRef]
- Knoop, R.J.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High molecular weight poly (ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Chebbi, Y.; Kasmi, N.; Majdoub, M.; Papageorgiou, G.; Achilias, D.; Bikiaris, D. Solid-state polymerization of poly (ethylene furanoate) biobased polyester, III: Extended study on effect of catalyst type on molecular weight increase. Polymers 2019, 11, 438. [Google Scholar] [CrossRef] [Green Version]
- Post drawing of sewing yarns—A problem of economically dealing with small lot sizes. Chem. Fibers. Int. 2000, 50, 190–191.
- Gries, T.; Veith, D.; Wulfhorst, B. Textile Fertigungsverfahren—Eine Einführung, 2nd ed.; Carl Hanser Verlag: München, Germany, 2014. [Google Scholar]
- Falkai, B.E. Synthesefasern Grundlagen, Technologie, Verarbeitung und Anwendung; Verlag Chemie: Weilheim, Germany; Deerfield Beach, IL, USA; Basel, Switzherland, 1981. [Google Scholar]
- Bauer, K.H. Streckspulmaschinen: Herstellung von Glattgarnen mit angepassten Eigenschaften auf Kreuzspulen für die textile und technische Anwendung. Chemiefasern Textilindustrie 1992, 42, 659–661. [Google Scholar]
- Schindler, S.; Bauder, H.-J.; Planck, H. Tailor-made special yarns from PET POY for technical and medical products. Chem. Fibers. Int. 2011, 4, 2014–2016. [Google Scholar]
- Shealy, O.L.; Kitson, R.E. An age-stable feed yarn for draw texturing by the false-twisting process. Text. Res. J. 1975, 45, 112–117. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, S.; Ren, M.; Xiao, C. Model of cold crystallization of uniaxially oriented poly (ethylene terephthalate) fibers. Polymer 2004, 45, 4361–4365. [Google Scholar] [CrossRef]
- Rabiei, N.; Kish, M.H.; Amirshahi, S.A. Monitoring the structural relaxation in poly (ethylene terephthalate) fibers: Dye sorptionand FTIR considerations. Polymer 2016, 106, 72–84. [Google Scholar] [CrossRef]
- Seyfart, E. Versuche zum alterungsverhalten ungereckter polyesterseiden. Faserforsch. Textiltechn. 1970, 21, 176–179. [Google Scholar]
- Hagege, R. Ageing behavior of pre-oriented PET yarns, followed by DSC. Text. Res. J. 1977, 47, 229–231. [Google Scholar]
- König, G. Streckzwirnen und streckspulen—Neue trends schaffen neue märkte. Chemiefasern Textilindustrie 1990, 40, 848–856. [Google Scholar]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully biobased superpolymers of 2,5-furandicarboxylic acid with different functional properties: From rigid to flexible, high performant packaging materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef]
- Konstantopoulou, M.; Terzopoulou, Z.; Nerantzaki, M.; Tsagkalias, J.; Achilias, D.S.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Poly (ethylene furanoate-co-ethylene terephthalate) biobased copolymers: Synthesis, thermal properties and cocrystallization behavior. Eur. Polym. J. 2017, 89, 349–366. [Google Scholar] [CrossRef]
- Lotti, N.; Munari, A.; Gigli, M.; Gazzano, M.; Tsanaktsis, V.; Bikiaris, D.N.; Papageorgiou, G.Z. Thermal and structural response of in situ prepared biobased poly (ethylene 2,5-furan dicarboxylate) nanocomposites. Polymer 2016, 103, 288–298. [Google Scholar] [CrossRef]
- Forestier, E.; Combeaud, C.; Guigo, N.; Monge, G.; Haudin, J.M.; Sbirrazzuoli, N.; Billon, N. Strain-induced crystallization of poly (ethylene 2,5-furandicarboxylate). Mechanical and crystallographic analysis. Polymer 2020, 187, 122126–122136. [Google Scholar] [CrossRef] [Green Version]
- Maini, L.; Gigli, M.; Gazzano, M.; Lotti, N.; Bikiaris, D.; Papageorgiou, G. Structural investigation of poly (ethylene furanoate) polymorphs. Polymers 2018, 10, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, L.; Niknam, V.; Guigo, N.; van Berkel, J.G.; Sbirrazzuoli, N. Morphology and thermal properties of novel clay-based poly (ethylene 2,5-furandicarboxylate) (PEF) nanocomposites. RSC Adv. 2016, 6, 59800–59807. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Papadopoulos, L.; Zamboulis, A.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Tuning the properties of furandicarboxylic acid-based polyesters with copolymerization: A review. Polymers 2020, 12, 1209. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Bikiaris, D.N.; Papageorgiou, G.Z. Crystallization and polymorphism of poly (ethylene furanoate). Crystal growth & design. Cryst. Growth Des. 2015, 15, 5505–5512. [Google Scholar]
- van Berkel, J.G.; Guigo, N.; Kolstad, J.J.; Sipos, L.; Wang, B.; Dam, M.A.; Sbirrazzuoli, N. Isothermal crystallization kinetics of poly (ethylene 2,5-furandicarboxylate). Macromol. Mater. Eng. 2015, 300, 466–474. [Google Scholar] [CrossRef]
- Righetti, M.C.; Marchese, P.; Vannini, M.; Celli, A.; Lorenzetti, C.; Cavallo, D.; Ocando, C.; Müller, A.J.; Androsch, R. Polymorphism and multiple melting behavior of bio-based poly (propylene 2,5-furandicarboxylate). Biomacromolecules 2020, 21, 2622–2634. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kasmi, N.; Siampani, M.; Terzopoulou, Z.; Bikiaris, D.; Achilias, D.; Papageorgiou, D.; Papageorgiou, G. Exploring next-generation engineering bioplastics: Poly (alkylene furanoate)/poly (alkylene terephthalate). Polymers 2019, 11, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoclet, G.; Gobius Du Sart, G.; Yeniad, B.; de Vos, S.; Lefebvre, J.M. Isothermal crystallization and structural characterization of poly (ethylene-2,5-furanoate). Polymers 2015, 72, 165–176. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. A Polym. Chem. 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H.; Wu, L.; Li, B.-G.; Dubois, P. DBU-catalyzed biobased poly (ethylene 2,5-furandicarboxylate) polyester with rapid melt crystallization: Synthesis, crystallization kinetics and melting behavior. RSC Adv. 2016, 6, 101578–101586. [Google Scholar] [CrossRef]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A perspective on PEF synthesis, properties, and end-life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef]
- Daubeny, R.D.P.; Bunn, C.W. The crystal structure of polyethylene terephthalate. Proc. R.Soc. Lond. Ser. A Math. Phys. Sci. 1954, 226, 531–542. [Google Scholar]
- Wunderlich, B. The ATHAS database on heat capacities of polymers. Pure Appl. Chem. 1994, 67, 1019–1026. [Google Scholar] [CrossRef]
- Codou, A.; Guigo, N.; van Berkel, J.; de Jong, E.; Sbirrazzuoli, N. Non-isothermal crystallization kinetics of biobased poly (ethylene 2,5-furandicarboxylate) synthesized via the direct esterification process. Macromol. Chem. Phys. 2014, 215, 2065–2074. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, P. Crystallization of biodegradable and biobased polyesters: Polymorphism, cocrystallization, and structure-property relationship. Prog. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Abasi, M.; Kotek, R. Effects of drawing process on crimp formation-ability of side-by-sidebicomponent filament yarns produced from recycled, fiber-grade andbottle-grade PET. J. Text. Inst. 2019, 110, 1439–1444. [Google Scholar] [CrossRef]
- Technology and Markets Day Path to the Future. Avantium. 2019. Available online: https://www.avantium.com/wp-content/uploads/2019/06/20190606-Technology-Day_CTO_Gert-Jan_Gruter_breakout_final_.pdf (accessed on 10 November 2020).
- Rosenboom, J.G.; Hohl, D.K.; Fleckenstein, P.; Storti, G.; Morbidelli, M. Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers. Nat. Commun. 2018, 9, 2701. [Google Scholar] [CrossRef] [Green Version]
- Banella, M.B.; Bonucci, J.; Vannini, M.; Marchese, P.; Lorenzetti, C.; Celli, A. Insights into the synthesis of poly (ethylene 2,5-furandicarboxylate) from 2,5-furandicarboxylic acid: Steps toward environmental and food safety excellence in packaging applications. Industr. Eng. Chem. Res. 2019, 58, 8955–8962. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Engel, C.A.R.; van der Meer, J.; Geers, L.F.G.; Crockatt, M. Method for Purifying and Removing Color of 2,5-Furandicarboxylic Acid (FDCA). WIPO (PCT). W.O. Patent 2,019,132,663 A1, 29 December 2017. [Google Scholar]
- Zou, X.; Zhu, C.; Wang, Q.; Yang, G. Catalytic dehydration of hexose sugars to 5-hydroxymethylfural. Biofuels Bioprod. Biorefining 2019, 13, 153–173. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Regestein, L.; Klement, T.; Grande, P.; Kreyenschulte, D.; Heyman, B.; Maßmann, T.; Eggert, A.; Sengpiel, R.; Wang, Y.; Wierckx, N.; et al. From beech wood to itaconic acid: Case study on biorefinery process integration. Biotechnol. Biofuels 2018, 11, 279. [Google Scholar] [CrossRef]
- Raab, T.; López-Ráez, J.A.; Klein, D.; Caballero, J.L.; Moyano, E.; Schwab, W.; Muñoz-Blanco, J. FaQR, required for the biosynthesis of the strawberry flavor compound 4-hydroxy-2, 5-dimethyl-3 (2H)-furanone, encodes an enone oxidoreductase. Plant Cell Environ. 2006, 18, 1023–1037. [Google Scholar] [CrossRef] [Green Version]
- Carsten, J.M.; Schmidt, A.; Sieber, V. Characterization of recombinantly expressed dihydroxy-acid dehydratase from Sulfobus solfataricus—A key enzyme for the conversion of carbohydrates into chemicals. J. Biotechnol. 2015, 211, 31–41. [Google Scholar] [CrossRef]
- Pick, A.; Schmid, J.; Sieber, V. Characterization of uronate dehydrogenases catalysing the initial step in an oxidative pathway. Microb. Biotechnol. 2015, 8, 633–643. [Google Scholar] [CrossRef]
- Sperl, J.M.; Carsten, J.M.; Guterl, J.-K.; Lommes, P.; Sieber, V. Reaction design for the compartmented combination of heterogeneous and enzyme catalysis. ACS Catal. 2016, 6, 6329–6334. [Google Scholar] [CrossRef]
- Guterl, J.K.; Garbe, D.; Carsten, J.; Steffler, F.; Sommer, B.; Reiße, S.; Philipp, A.; Haack, M.; Rühmann, B.; Koltermann, A. Cell-free metabolic engineering: Production of chemicals by minimized reaction cascades. ChemSusChem 2012, 5, 2165–2172. [Google Scholar] [CrossRef]
- Sieber, V.; Pick, A.; Rühmann, B. Synthetic Pathway for the Production of Alcohols or Amines. European E.P. Patent 2,503,003 A1, 24 March 2011. [Google Scholar]
- Siegrist, J.; Aschwanden, S.; Mordhorst, S.; Thöny-Meyer, L.; Richter, M.; Andexer, J.N. Regiocomplementary O-methylation of catechols by using three-enzyme cascades. Chembiochem 2015, 16, 2576–2579. [Google Scholar] [CrossRef]
- Rim, P.B.; Nelson, C.J. Properties of PET fibers with high modulus and low shrinkage (HMLS). I. yarn properties and morphology. J. Appl. Polym. Sci. 1991, 42, 1807–1813. [Google Scholar] [CrossRef]
- Kramer, T.; Reese, W.; Justine, C. Reinforcement Cord For Elastomer Products, in Particular for a Motor Vehicle Air Tire, and Motor Vehicle Air Tire. WIPO (PCT). W.O. Patent 2,014,040,804 A1, 12 September 2012. [Google Scholar]
- Chung, I.; Jeon, O.-H.; Kim, G.-W. Undrawn Polyethylene Terephthalate (PET) Fiber, Drawn PET Fiber, and Tire-Cord Comprising the Same United States Patent. U.S. Patent 9,005,754 B2, 14 April 2015. [Google Scholar]
- Forestier, E.; Combeauda, E.; Guigo, N.; Sbirrazzuolib, N.; Billona, N. Understanding of strain-induced crystallization developments scenarios for polyesters: Comparison of poly (ethylene furanoate), PEF, and poly (ethylene terephthalate), PET. Polymer 2020, 203, 122755. [Google Scholar] [CrossRef]
- Kim, J.H.; Yang, S.S.; Hudson, S.M. Comparison of the structure-property relationships for PTT and PET fibers spun at various take-up speeds. Fibers Poym. 2011, 12, 771–777. [Google Scholar] [CrossRef]





| Sample | Monomer/Route | [η] (before SSP)/dL·g−1 | CEG (before SSP)/µmol·g−1 | Mn (before SSP)/g·mol−1 | Đ (before SSP)- | [η] (after SSP)/dL·g−1 | Mn (after SSP)/g·mol−1 | Đ (after SSP) /- |
|---|---|---|---|---|---|---|---|---|
| PEF-01 | FDME | 0.536 | 37 | 22,500 | 2.9 | 0.572 | 35,700 | 2.6 |
| PEF-02 | FDME | 0.588 | 57 | 34,600 | 2.6 | 0.655 | 35,900 | 3.3 |
| PEF-03 | FDME | 0.626 | 50 | 37,200 | 2.6 | 0.747 | 53,900 | 2.6 |
| PEF-04 | FDME | 0.613 | 53 | 38,500 | 2.4 | 0.780 | 50,400 | 2.6 |
| PEF-05 | FDME | 0.598 | 55 | 31,900 | 2.8 | 0.790 | 39,700 | 3.3 |
| PEF-06 | FDME | 0.601 | 56 | 26,600 | 2.7 | 0.780 | 29,000 | 4.0 |
| PEF-07 | FDCA(Holypharm) | 0.398 | 7 | 24,600 | 2.6 | 0.446 | 24,000 | 2.9 |
| PEF-08 | FDCA(Purac) | 0.600 | 21 | 46,600 | 2.3 | 0.850 | 55,900 | 3.0 |
| Sample | Monomer/Route | Tg /°C | Tm,p /°C | ΔHm /J·g−1 | χc 1 /% | χc 2 /% |
|---|---|---|---|---|---|---|
| PEF-03 | FDME | 81.4 | 215.4 | 66.7 | 48.7 | 43 |
| PEF-04 | FDME | 91.4 | 214.6 | 63.2 | 46.1 | 43 |
| Sample | Spinning Temp. /°C | Winder /m·min−1 | Titer /dtex | Tg /°C | Tm,p /°C | ΔHm /J·g−1 |
|---|---|---|---|---|---|---|
| LOY-03a 1 | 260 | 500 | 1260 | 91.0 | - 3 | 0 3 |
| LOY-03b 2 | 260 | 500 | 1260 | 88.7 | - 3 | 0 3 |
| LOY-04 | 260 | 500 | 1240 | 90.2 | 204.8 | 1.3 |
| POY-03 | 260 | 2500 | 133 | 88.8 | 206.6 | 31.3 |
| POY-04 | 260 | 2200 | 143 | 89.5 | 204.5 | 34.5 |
| POY-05 | 275 | 2200 | 149 | 89.4 | 203.7 | 31.9 |
| Sample | DR /- | Tt /dtex | Tg /°C | Tm,p /°C | ΔHm /J·g−1 | σm /cN·tex−1 | εB /% | E /cN·tex−1 |
|---|---|---|---|---|---|---|---|---|
| FDY-L-04 | 2.73 | 463 | 90.0 | 203.4 | 41.7 | 34.4 ± 1.1 | 7.4 ± 2.9 | 990 ± 20 |
| FDY-P-03 | 1.99 | 68 | 90.0 | 201.3 | 47.3 | 63.0 ± 3.0 | 4.7 ± 0.2 | 1510 ± 60 |
| FDY-P-04 | 1.81 | 80 | 90.1 | 204.9 | 48.3 | 54.4 ± 1.8 | 5.1 ± 0.3 | 1270 ± 75 |
| FDY-P-05 | 1.89 | 79 | 89.9 | 204.9 | 48.8 | 65.2 ±0.8 | 6.0 ± 0.1 | 1370 ± 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höhnemann, T.; Steinmann, M.; Schindler, S.; Hoss, M.; König, S.; Ota, A.; Dauner, M.; Buchmeiser, M.R. Poly(Ethylene Furanoate) along Its Life-Cycle from a Polycondensation Approach to High-Performance Yarn and Its Recyclate. Materials 2021, 14, 1044. https://doi.org/10.3390/ma14041044
Höhnemann T, Steinmann M, Schindler S, Hoss M, König S, Ota A, Dauner M, Buchmeiser MR. Poly(Ethylene Furanoate) along Its Life-Cycle from a Polycondensation Approach to High-Performance Yarn and Its Recyclate. Materials. 2021; 14(4):1044. https://doi.org/10.3390/ma14041044
Chicago/Turabian StyleHöhnemann, Tim, Mark Steinmann, Stefan Schindler, Martin Hoss, Simon König, Antje Ota, Martin Dauner, and Michael R. Buchmeiser. 2021. "Poly(Ethylene Furanoate) along Its Life-Cycle from a Polycondensation Approach to High-Performance Yarn and Its Recyclate" Materials 14, no. 4: 1044. https://doi.org/10.3390/ma14041044
APA StyleHöhnemann, T., Steinmann, M., Schindler, S., Hoss, M., König, S., Ota, A., Dauner, M., & Buchmeiser, M. R. (2021). Poly(Ethylene Furanoate) along Its Life-Cycle from a Polycondensation Approach to High-Performance Yarn and Its Recyclate. Materials, 14(4), 1044. https://doi.org/10.3390/ma14041044