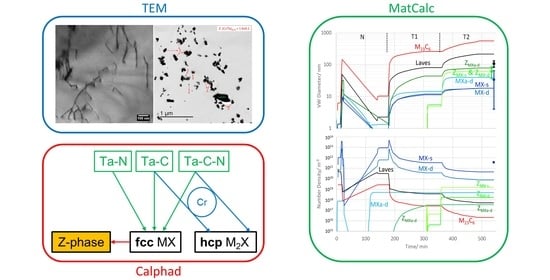
Thermodynamic Modelling and Microstructural Study of Z-Phase Formation in a Ta-Alloyed Martensitic Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Incorporation of Ta into Thermodynamic and Diffusion Mobility Databases
4. Input Parameters for Thermokinetic Simulations
4.1. Chemistry
4.2. Simulated Precipitate Phases
4.3. Simulation Modes
4.4. Nucleation Sites
4.5. Z-Phase Transformation Model
4.6. Heat Treatment
4.7. Microstructural Settings
4.8. Z-Phase Settings
5. Results of the TEM Measurements
5.1. Precipitates
5.2. Dislocation Densities and Subgrain Sizes
6. Results of MatCalc Simulations
6.1. Equilibrium ZULC and Z6
6.2. Simulation of Dislocation Evolution
6.3. Precipitate Kinetic Simulation
6.3.1. Z6, Heat Treatment Up to Condition 1 (N + T)
6.3.2. Z6, Ageing Up to Condition 2
7. Discussion
7.1. Z-Phase Precursors MX
7.2. Z-Phase Precursors M2X
7.3. Z-Phase Size Evolution
7.4. Chemical Composition of Z-Phase Precipitates
7.5. Calibration of Transformation Model
7.6. Overall Number Densities and Size Distributions
7.7. C Content and Amount of M23C6
7.8. BN Implications
8. Conclusions
- Element Ta and its interactions with Cr, N and C—determining the nucleation behaviour of MX precursors and enabling Z-phase to form—have been successfully implemented into the thermodynamic steel database mc_fe.
- The dissolution temperature of Ta-based Z-phase contributed to the parametrization of the thermodynamic model.
- A model based on inner-particle nucleation was applied for the transformation of metastable MX precursors into Z-phase controlled by Cr intake.
- The parameter setup for the thermokinetic calculation involves detailed microstructural input data—in particular dislocation densities—which were gained from TEM measurements and combined with modelling of dislocation evolution.
- The simulation results were validated based on our TEM precipitate results as well as APT data from the literature. Modelled Z-phase size, number density and chemical composition showed excellent agreement to measurements. The simulation greatly contributed to the interpretation of the experimental results from TEM analysis (especially to the size distributions).
- Thermokinetic simulation tools as presented here can assist improved engineering of novel creep-resistant materials to make thermal power plant operation safer, more predictable and more efficient.
9. Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeiler, G. Martensitic Steels for Rotors in Ultra-Supercritical Power Plants. In Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Di Gianfrancesco, A., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 143–174. ISBN 978-0-08-100552-1. [Google Scholar]
- Hald, J. Development status und future possibilities for martensitic creep resistant steels. In Proceedings of the 9th Liége Conference: Materials for Advanced Power Engineering, Liege, Belgium, 27–29 September 2010; pp. 53–64. [Google Scholar]
- Raj, B.; Choudhary, B.K. A perspective on creep and fatigue issues in sodium cooled fast reactors. Trans. Indian Inst. Met. 2010, 63, 75–84. [Google Scholar] [CrossRef]
- Kalck, C.; Fournier, B.; Barcelo, F.; Forest, L.; Dalle, F.; Giroux, P.-F.; Tournié, I.; Gourgues-Lorenzon, A.-F. High temperature creep properties and microstructural examinations of P92 welds. In Proceedings of the 9th Liége Conference: Materials for Advance Power Engineering, Liege, Belgium, 27–29 September 2010; pp. 424–434. [Google Scholar]
- Viswanathan, R.; Gandy, D.; Coleman, K. Advances in Materials Technology for Fossil Power Plants. In Proceedings of the 4th International Conference, Hilton Head Island, SC, USA, 25–28 October 2004; pp. 37–40. [Google Scholar]
- Hald, J. Microstructure and long-term creep properties of 9–12% Cr steels. Int. J. Pres. Ves. Pip. 2008, 85, 30–37. [Google Scholar] [CrossRef]
- Abe, F. Grade 91 Heat-Resistant Martensitic Steel. In Coal Power Plant Materials and Life Assessment; Shibli, A., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 3–51. ISBN 978-0-85709-431-5. [Google Scholar]
- Maruyama, K.; Sawada, K.; Koike, J. Strengthening Mechanisms of Creep Resistant Tempered Martensitic Steel. ISIJ Int. 2001, 41, 13. [Google Scholar] [CrossRef]
- Pandey, C.; Giri, A.; Mahapatra, M.M. Evolution of phases in P91 steel in various heat treatment conditions and their effect on microstructure stability and mechanical properties. Mater. Sci. Eng. 2016, 664, 58–74. [Google Scholar] [CrossRef]
- Cerri, E.; Evangelista, E.; Spigarelli, S.; Bianchi, P. Evolution of microstructure in a modified 9Cr–1Mo steel during short term creep. Mater. Sci. Eng. 1998, 245, 285–292. [Google Scholar] [CrossRef]
- Gustafson, Å.; Hättestrand, M. Coarsening of precipitates in an advanced creep resistant 9% chromium steel—Quantitative microscopy and simulations. Mater. Sci. Eng. 2002, 333, 279–286. [Google Scholar] [CrossRef]
- Panait, C.G.; Zielińska-Lipiec, A.; Koziel, T.; Czyrska-Filemonowicz, A.; Gourgues-Lorenzon, A.-F.; Bendick, W. Evolution of dislocation density, size of subgrains and MX-type precipitates in a P91 steel during creep and during thermal ageing at 600 °C for more than 100,000 h. Mater. Sci. Eng. 2010, 527, 4062–4069. [Google Scholar] [CrossRef] [Green Version]
- Panait, C.G.; Bendick, W.; Fuchsmann, A.; Gourgues-Lorenzon, A.-F.; Besson, J. Study of the microstructure of the Grade 91 steel after more than 100,000 h of creep exposure at 600 °C. Int. J. Pres. Ves. Pip. 2010, 87, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Cipolla, L.; Danielsen, H.K.; Venditti, D.; Di Nunzio, P.E.; Hald, J.; Somers, M.A.J. Conversion of MX nitrides to Z-phase in a martensitic 12% Cr steel. Acta Mater. 2010, 58, 669–679. [Google Scholar] [CrossRef]
- Danielsen, H.K. Review of Z phase precipitation in 9–12 wt-% Cr steels. Mater. Sci. Tech. 2016, 32, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Danielsen, H.K.; Hald, J. Tantalum-containing Z-phase in 12%Cr martensitic steels. Scr. Mater. 2009, 60, 811–813. [Google Scholar] [CrossRef]
- Liu, F.; Rashidi, M.; Hald, J.; Reißig, L.; Andrén, H.O. Microstructure of Z-Phase Strengthened Martensitic Steels: Meeting the 650 °C Challenge. Mater. Sci. Forum 2017, 879, 1147–1152. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, M.; Johansson, L.; Andrén, H.-O.; Liu, F. Microstructure and mechanical properties of two Z-phase strengthened 12%Cr martensitic steels: The effects of Cu and C. Mater. Sci. Eng. 2017, 694, 57–65. [Google Scholar] [CrossRef]
- Rashidi, M.; Odqvist, J.; Johansson, L.; Hald, J.; Andrén, H.-O.; Liu, F. Experimental and theoretical investigation of precipitate coarsening rate in Z-phase strengthened steels. Materialia 2018, 4, 247–254. [Google Scholar] [CrossRef]
- Danielsen, H.K.; Hald, J. On the nucleation and dissolution process of Z-phase Cr(V,Nb)N in martensitic 12% Cr steels. Mater. Sci. Eng. 2009, 505, 169–177. [Google Scholar] [CrossRef]
- Agamennone, R.; Blum, W.; Gupta, C.; Chakravartty, J.K. Evolution of microstructure and deformation resistance in creep of tempered martensitic 9–12% Cr–2% W–5% Co steels. Acta Mater. 2006, 54, 3003–3014. [Google Scholar] [CrossRef]
- Rashidi, M.; Golpayegani, A.; Sheikh, S.; Guo, S.; Andrén, H.-O.; Liu, F. Mechanistic insights into the transformation processes in Z-phase strengthened 12% Cr steels. Mater. Des. 2018, 158, 237–247. [Google Scholar] [CrossRef]
- Vodárek, V.; Filuš, F. On the Role of Z-Phase in Heat Resistant Steels. In Proceedings of the Metal 2011: International Conference on Metallurgy and Materials, Brno, Czech Republic, 8–20 May 2011; pp. 1–6. [Google Scholar]
- Ettmayer, P. Die Struktur der Komplexnitride NbCrN und Ta1−xCr1+xN. Chem. Mon. 1971, 102, 858–863. [Google Scholar] [CrossRef]
- Liu, F.; Rashidi, M.; Johansson, L.; Hald, J.; Andrén, H.-O. A new 12% chromium steel strengthened by Z-phase precipitates. Scr. Mater. 2016, 113, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Sanhueza, J.P.; Rojas, D.; Prat, O.; García, J.; Meléndrez, M.F.; Suarez, S. Investigation of Ta-MX/Z-Phase and Laves Phase as Precipitation Hardening Particles in a 12 Pct Cr Heat-Resistant Steel. Metall. Mater. Trans. 2018, 49, 2951–2962. [Google Scholar] [CrossRef]
- MatCalc. The Materials Calculator. Available online: https://matcalc.at (accessed on 20 December 2020).
- MatCalc. Open Databases. Available online: https://matcalc.at/index.php/databases/open-databases (accessed on 20 December 2020).
- Tassa, O.; Matera, S.; Matocha, K.; Kuboň, Z.; Liška, M.; Kurka, V.; Gsellmann, B.; Sonderegger, B.; Iranshahi, F.; Riedlsperger, F.; et al. New Creep Resistant Stable Steel for USC Power Plant; Final Report of EU Project CRESTA2 (RFSR-CT-2014-00032); CSM: Rome, Italy, 2019. [Google Scholar]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Voblikov, V.M.; Fomichov, O.S.; Petyukh, V.M.; Rex, S. Experimental study and thermodynamic re-assessment of the binary Fe–Ta system. Intermetallics 2011, 19, 1059–1075. [Google Scholar] [CrossRef]
- Dupin, N.; Ansara, I. Thermodynamic assessment of the Cr-Ta system. J. Phase Equilib. 1993, 14, 451–456. [Google Scholar] [CrossRef]
- Schönberg, N. Ternary metallic phases in the Ta-C-N, Ta-C-O and Ta-N-O systems. Acta Chem. Scand. 1954, 8, 620–623. [Google Scholar] [CrossRef] [Green Version]
- Frisk, K. Analysis of the phase diagram and thermochemistry in the Ta–N and the Ta–C–N systems. J. Alloys Compd. 1998, 278, 216–226. [Google Scholar] [CrossRef]
- Grumski, M.; Dholabhai, P.P.; Adams, J.B. Ab initio study of the stable phases of 1:1 tantalum nitride. Acta Mater. 2013, 61, 3799–3807. [Google Scholar] [CrossRef]
- Li, D.; Tian, F.; Duan, D.; Bao, K.; Chu, B.; Sha, X.; Liu, B.; Cui, T. Mechanical and metallic properties of tantalum nitrides from first-principles calculations. RSC Adv. 2014, 4, 10133–10139. [Google Scholar] [CrossRef]
- Danielsen, H.K.; Hald, J. A thermodynamic model of the Z-phase Cr(V, Nb)N. Calphad 2007, 31, 505–514. [Google Scholar] [CrossRef]
- Sonderegger, B.; Kozeschnik, E. Size dependence of the interfacial energy in the generalized nearest-neighbor broken-bond approach. Scr. Mater. 2009, 60, 635–638. [Google Scholar] [CrossRef]
- Urban, D.F.; Elsässer, C. Atomic defects and dopants in ternary Z-phase transition-metal nitrides Cr M N with M = V, Nb, Ta investigated with density functional theory. Phys. Rev. 2017, 96, 104107. [Google Scholar] [CrossRef] [Green Version]
- Danielsen, H.K.; Hald, J.; Somers, M.A.J. Atomic resolution imaging of precipitate transformation from cubic TaN to tetragonal CrTaN. Scr. Mater. 2012, 66, 261–264. [Google Scholar] [CrossRef]
- Fridberg, J.; Torndahl, L.; Hillert, M. Diffusion in Iron. Jernkontorets Ann. 1969, 153, 263–276. [Google Scholar]
- Russell, K.C. Nucleation in solids: The induction and steady state effects. Adv. Colloid Interface Sci. 1980, 13, 205–318. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal Relations in Irreversible Processes. I. Phys. Rev. 1931, 37, 405–426. [Google Scholar] [CrossRef]
- Svoboda, J.; Fischer, F.; Fratzl, P.; Kozeschnik, E. Modelling of kinetics in multi-component multi-phase systems with spherical precipitates I: Theory. Mater. Sci. Eng. 2004, 385, 166–174. [Google Scholar] [CrossRef]
- Sonderegger, B.; Kozeschnik, E. Generalized Nearest-Neighbor Broken-Bond Analysis of Randomly Oriented Coherent Interfaces in Multicomponent Fcc and Bcc Structures. Metall. Mater. Trans. 2009, 40, 499–510. [Google Scholar] [CrossRef]
- Srinivas Prasad, B.S.; Rajkumar, V.B.; Kumar, H. Numerical simulation of precipitate evolution in ferritic–martensitic power plant steels. Calphad 2012, 36, 1–7. [Google Scholar] [CrossRef]
- Kozeschnik, E.; Warczok, P. Treatment of Heterogeneous Nucleation; in MatCalc Documentation. Available online: https://www.matcalc.at/wiki/doku.php?id=techpapers:nucleation:het_nucl_sites (accessed on 20 December 2020).
- Janssens, K.G.F.; Raabe, D.; Kozeschnik, E.; Miodownik, M.A.; Nestler, B. Computational Materials Engineering: An Introduction to Microstructure Evolution; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Radis, R.; Schaffer, M.; Albu, M.; Kothleitner, G.; Pölt, P.; Kozeschnik, E. Multimodal size distributions of γ’ precipitates during continuous cooling of UDIMET 720 Li. Acta Mater. 2009, 57, 5739–5747. [Google Scholar] [CrossRef]
- Zhou, T.; Babu, R.P.; Hou, Z.; Odqvist, J.; Hedström, P. Precipitation of multiple carbides in martensitic CrMoV steels—Experimental analysis and exploration of alloying strategy through thermodynamic calculations. Materialia 2020, 9, 100630. [Google Scholar] [CrossRef]
- Buken, H.; Sherstnev, P.; Kozeschnik, E. A state parameter-based model for static recrystallization interacting with precipitation. Model. Simul. Mater. Sci. Eng. 2016, 24, 35006. [Google Scholar] [CrossRef]
- Zamberger, S.; Whitmore, L.; Krisam, S.; Wojcik, T.; Kozeschnik, E. Experimental and computational study of cementite precipitation in tempered martensite. Model. Simul. Mater. Sci. Eng. 2015, 23, 55012. [Google Scholar] [CrossRef]
- Nitta, H.; Miura, K.; Iijima, Y. Self-Diffusion in Iron-Based Fe–Mo Alloys. Acta Mater. 2006, 54, 2833–2847. [Google Scholar] [CrossRef]
- Danielsen, H.K.; Hald, J.; Grumsen, F.B.; Somers, M.A.J. On the crystal structure of Z-phase Cr(V,Nb)N. Metall. Mater. Trans. 2006, 37, 2633–2640. [Google Scholar] [CrossRef]
- Sonderegger, B. Modifications of Stereological Correction Methods for Precipitate Parameters Using Transmission Microscopy. Ultramicroscopy 2006, 106, 941–950. [Google Scholar] [CrossRef]
- Kenik, E.A.; Maziasz, P.J. Application of Extraction Replicas and Analytical Electron Microscopy to Precipitate Phase Studies. Report of Oak Ridge National Laboratory 1984. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/16/033/16033847.pdf?r=1 (accessed on 8 January 2021).
- Di Nunzio, P.E. Reinterpretation of the Mean Field Hypothesis in Analytical Models of Ostwald Ripening and Grain Growth. Metall. Mater. Trans. 2019, 50, 2066–2080. [Google Scholar] [CrossRef]
- Di Nunzio, P.E. A Discrete Model of Ostwald Ripening Based on Multiple Pairwise Interactions. Philos. Mag. 2018, 98, 1674–1695. [Google Scholar] [CrossRef]
- Abe, F. Effect of boron on microstructure and creep strength of advanced ferritic power plant steels. Procedia Eng. 2011, 10, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Sakuraya, K.; Okada, H.; Abe, F. BN type inclusions formed in high Cr ferritic heat resistant steel. Energy Mater. 2006, 1, 158–166. [Google Scholar] [CrossRef]
- Riedlsperger, F.; Krenmayr, B.; Zuderstorfer, G.; Fercher, B.; Niederl, B.; Schmid, J.; Sonderegger, B. Application of an advanced mean-field dislocation creep model to P91 for calculation of creep curves and time-to-rupture diagrams. Materialia 2020, 12, 100760. [Google Scholar] [CrossRef]
















| wt.% | Fe | Ni | Cu | Cr | W | Mo | Si |
|---|---|---|---|---|---|---|---|
| ZULC | bal | 0.50 | - | 11.79 | 2.90 | - | 0.30 |
| Z6 | bal | 0.22 | 0.98 | 10.90 | 1.70 | 0.74 | 0.05 |
| wt.% | Mn | C | N | Co | Ta | B | V |
| ZULC | 0.48 | 0.005 | 0.033 | 7.30 | 0.39 | 0.004 | - |
| Z6 | 0.49 | 0.009–0.017 | 0.035 | 3.71 | 0.38 | 0.003 | 0.013 |
| BCC_A2 | : Co,Cr,Cu,Fe%,Mn,Mo,Ni,Si,Ta,V,W : B,C,N,Va% : |
| Interaction Parameters | |
| FCC_A1 | : Co,Cr,Cu,Fe%,Mn,Mo,Ni,Si,Ta,V,W : B,C,N,Va% : |
| Interaction Parameters | |
| HCP_A3 | : Co,Cr,Cu,Fe%,Mn,Mo,Ni,Si,Ta,V,W : B,C,N,Va% : |
| Interaction Parameters | |
| Z-Phase | : Cr%,Fe : Cr,Nb,Mo,Ta,V : N%,Va : |
| Interaction Parameters | |
| Nucleation Sites Z6 | MX | M23C6 | Laves | Z | |
|---|---|---|---|---|---|
| Matrix | Austenite | d | - | - | - |
| Martensite | d, s | s | g | MXi | |
| g = Grain Boundaries; d = Dislocations; s = Subgrain Boundaries; MXi = MX as Z-phase Nucleation Sites (On-Particle Nucleation) | |||||
| Input MatCalc | Value | Source | ||
|---|---|---|---|---|
| Databases | mc_fe_v2.061.tdb and mc_fe_v2.013.ddb | This work | ||
| Heat Treatment | Normalizing | 1 h @ 1100 °C | [29] | |
| Tempering | 2 h @ 650 °C & 2 h @ 750 °C | |||
| Ageing | 1000 h @ 700 °C | |||
| PAGS | 48 µm | [29] | ||
| Subgrain Size | 0.41 µm | TEM | ||
| Dislocation Density | Austenite | 1 × 1011 m−2 | [45] | |
| Fresh Martensite | 6.0 × 1014 m−2 | Equation (3) C = 3 × 10−4 | [50] calibrated by TEM (this work) | |
| Tempered Martensite | 2.6 × 1014 m−2 | |||
| Ageing | Start: 2.6 × 1014 m−2 End: 8 × 1013 m−2 | |||
| Martensite Start Temperature | 420 °C | [29] | ||
| Reaustenitization Temperature | 820 °C | [29] | ||
| Z-Phase | 0.22 Jm−2 | Fit | ||
| mnr | 5 Å | [16,53] | ||
| Cond. | Prec. | Di/nm | NEDX | NV/m−3 | NSize |
|---|---|---|---|---|---|
| 1 | MX | 34 ± 30 | 64 | 3.7 × 1021 | 4246 |
| Z | 61 ± 25 | 34 | |||
| Laves | 108 ± 24 | 5 | |||
| 2 | Z | 79 ± 38 | 88 | 2 × 1020 | 2434 |
| Laves | 551 ± 270 | 32 | |||
| M23C6 | 2728 ± 954 | 6 |
| Cond. | Microstructure | ρint [m−2] | Dsgb [µm] |
|---|---|---|---|
| 0 | Martensite | 6.0 ± 0.5 × 1014 | 0.48 ± 0.09 |
| 1 | Tempered Martens. | 2.6 ± 0.6 × 1014 | 0.41 ± 0.18 |
| 2 | Aged Temp. Martens. | 7.9 ± 2.1 × 1013 | 0.43 ± 0.14 |
| Material | Precipitate | 550 °C | 600 °C | 650 °C | 700 °C |
|---|---|---|---|---|---|
| [mol.%] | [mol.%] | [mol.%] | [mol.%] | ||
| ZULC | Laves | 2.54 | 2.43 | 2.26 | 1.99 |
| Z-phase | 0.41 | 0.41 | 0.41 | 0.41 | |
| M23C6 | 0.12 | 0.11 | 0.11 | 0.11 | |
| BN | 0.04 | 0.04 | 0.04 | 0.04 | |
| Z6 | Laves | 2.36 | 2.10 | 1.71 | 1.16 |
| Z-phase | 0.42 | 0.42 | 0.42 | 0.41 | |
| M23C6 | 0.20–0.39 | 0.20–0.39 | 0.20–0.38 | 0.20–0.38 | |
| MX (fcc) | None | 0.02 | 0.02 | 0.02 | |
| Cr2N (hcp) | 0.02 | None | None | None | |
| BN | 0.03 | 0.03 | 0.03 | 0.03 |
| MX Simulation Z6 (MatCalc) | MX Literature (APT) | |||||||||||
| Cond. | Chem. Comp. [at.%] | Cond. | Chem. Comp. [at.%] | Type | Ref. | |||||||
| Cr | N | Ta | V | C | Cr | N | Ta | C | ||||
| 80 min N | 7.2 | 26.6 | 42.2 | 0.0 | 23.3 | As-rec. | 42.6 | 45.9 | 6.6 | 0.4 | A | [18] |
| 35.8 | 48.1 | 9.3 | 0.8 | A | [22] | |||||||
| As-rec. (N + T) | 26.7 | 50.0 | 22.9 | 0.3 | 0.0 | 17.2 | 13.8 | 27.1 | 32.6 | B | [18] | |
| 18.4 | 29.4 | 27.0 | 13.3 | B | [22] | |||||||
| MX Measured (TEM-EDX) | ||||||||||||
| Cond. | Chem. Comp. [at.%] | |||||||||||
| Cr | Fe | Ta | V | W | ||||||||
| As-rec. | 14 ± 5 | 3 ± 2 | 75 ± 9 | <1 | 5 ± 3 | |||||||
| Z-Phase Simulation Z6 (MatCalc) | Z-Phase Literature (APT) | |||||||||
| Cond. | Chem. Comp. [at.%] | Cond. | Chem. Comp. [at.%] | Ref. | ||||||
| Cr | N | Ta | V | Cr | N | Ta | ||||
| As-rec. | 36.9 | 27.3 | 35.7 | 0.1 | As-rec. | 33.6 | 26.9 | 30.2 | [18] | |
| 103 h/700 °C | 40.7 | 28.7 | 28.4 | 2.2 | 104 h/650 °C | 39.8 | 30.2 | 24.1 | [18] | |
| Z-Phase Measured (TEM-EDX) | ||||||||||
| Cond. | Chem. Comp. [at.%] | |||||||||
| Cr | Fe | Ta | V | W | ||||||
| As-rec. | 51 ± 7 | 6 ± 2 | 37 ± 8 | 2 ± 1 | 3 ± 2 | |||||
| 103 h/700 °C | 52 ± 4 | 5 ± 2 | 36 ± 3 | 2 ± 1 | 3 ± 1 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedlsperger, F.; Gsellmann, B.; Povoden-Karadeniz, E.; Tassa, O.; Matera, S.; Dománková, M.; Kauffmann, F.; Kozeschnik, E.; Sonderegger, B. Thermodynamic Modelling and Microstructural Study of Z-Phase Formation in a Ta-Alloyed Martensitic Steel. Materials 2021, 14, 1332. https://doi.org/10.3390/ma14061332
Riedlsperger F, Gsellmann B, Povoden-Karadeniz E, Tassa O, Matera S, Dománková M, Kauffmann F, Kozeschnik E, Sonderegger B. Thermodynamic Modelling and Microstructural Study of Z-Phase Formation in a Ta-Alloyed Martensitic Steel. Materials. 2021; 14(6):1332. https://doi.org/10.3390/ma14061332
Chicago/Turabian StyleRiedlsperger, Florian, Bernadette Gsellmann, Erwin Povoden-Karadeniz, Oriana Tassa, Susanna Matera, Mária Dománková, Florian Kauffmann, Ernst Kozeschnik, and Bernhard Sonderegger. 2021. "Thermodynamic Modelling and Microstructural Study of Z-Phase Formation in a Ta-Alloyed Martensitic Steel" Materials 14, no. 6: 1332. https://doi.org/10.3390/ma14061332
APA StyleRiedlsperger, F., Gsellmann, B., Povoden-Karadeniz, E., Tassa, O., Matera, S., Dománková, M., Kauffmann, F., Kozeschnik, E., & Sonderegger, B. (2021). Thermodynamic Modelling and Microstructural Study of Z-Phase Formation in a Ta-Alloyed Martensitic Steel. Materials, 14(6), 1332. https://doi.org/10.3390/ma14061332