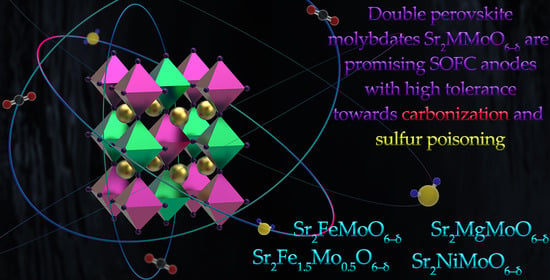
Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells
Abstract
:1. Introduction
2. Brief Descriptions of SOFCs
3. Anode Materials for SOFCs
- High catalytic activity in relation to fuel oxidation. In the case of hydrocarbon fuels, the catalytic properties of the material are preliminarily studied before using it as an anode. This is done to determine the mechanism and degree of fuel oxidation, synthesis gas yield and the possibility of carbon deposition after tests [36,41,42,45,47].
- Sufficient electron conductivity. Electrons formed as a result of electrochemical reactions at anode/electrolyte interface must be transported to the external circuit not only through current collectors, but also through the supported anode with high conductivity to suppress any unwanted ohmic losses in the electrodes.
- Thermal compatibility. The thermal expansion of the anode must be consistent with similar behavior in both the electrolyte and the current collector. This is required to prevent cracking between SOFC components during operation, heating, cooling or thermal cycling.
- Chemical stability. The anode must be chemically stable at operating temperatures not only in oxidizing and reducing atmospheres, but also in relation to the electrolyte and the current collector. Otherwise, the resulting impurities can block the transfer of electrons or oxygen ions along the corresponding paths in the SOFCs. It should be noted that chemical stability must also be verified by sintering the SOFCs, where temperatures are higher compared to working temperatures.
- Porosity. Since the fuel is a gas that must reach a triple-phase boundary (TPB) or the surface of a mixed-ionic conductor, the anode must exhibit a porous structure that retains its natural microstructural characteristics over a prolonged operating period.
4. General Features of Sr2MMoO6 (M = Ni, Mg, Fe)
5. Functional Properties of Sr2MMoO6 (M = Ni, Mg, Fe)
5.1. Sr2NiMoO6-δ
5.2. Sr2MgMoO6-δ
5.3. Sr2FeMoO6-δ
5.4. Sr2Fe1.5Mo0.5O6-δ
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Da Silva, F.S.; de Souza, T.M. Novel materials for solid oxide fuel cell technologies: A literature review. Int. J. Hydrog. Energy 2017, 42, 26020–26036. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, S. Solid oxide fuel cell: Materials for anode, cathode and electrolyte. Int. J. Hydrog. Energy 2020, 45, 23988–24013. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Lyskov, N.V.; Mazo, G.N.; Antipov, E.V. Electrode materials based on complex d-metal oxides for symmetrical solid oxide fuel cell. Russ. Chem. Rev. 2021, 90. [Google Scholar] [CrossRef]
- Khan, M.S.; Lee, S.B.; Song, R.H.; Lee, J.W.; Lim, T.H.; Park, S.J. Fundamental mechanisms involved in the degradation of nickel-yttria stabilized zirconia (Ni-YSZ) anode during solid oxide fuel cells. Ceram. Int. 2015, 42, 35–48. [Google Scholar] [CrossRef]
- Boldrin, P.; Ruiz-Trejo, E.; Mermelstein, J.; Menendez, J.M.B.; Reina, T.R.; Brandon, N.P. Strategies for carbon and sulfur tolerant solid oxide fuel cell materials, incorporating lessons from heterogeneous catalysis. Chem. Rev. 2016, 116, 13633–13684. [Google Scholar] [CrossRef]
- Gur, T.M. Comprehensive review of methane conversion in solid oxide fuel cells: Prospects for efficient electricity generation from natural gas. Prog. Energy Combust. Sci. 2016, 54, 1–64. [Google Scholar] [CrossRef] [Green Version]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Jablonski, W.S.; Villano, S.M.; Dean, A.M. A comparison of H2S, SO2, and COS poisoning on Ni/YSZ and Ni/K2O-CaAl2O4 during methane steam and dry reforming. Appl. Catal. A 2016, 502, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Lee, H.M.; Park, J.Y.; Lim, H.T. Degradation behavior of Ni-YSZ anode-supported solid oxide fuel cell (SOFC) as a function of H2S concentration. Int. J. Hydrog. Energy 2018, 43, 22511–22518. [Google Scholar] [CrossRef]
- Takeguchi, T.; Kani, Y.; Yano, T.; Kikuchi, R.; Eguchi, K.; Tsujimoto, K.; Uchida, Y.; Ueno, A.; Omoshiki, K.; Aizawa, M. Study on steam reforming of CH4 and C2 hydrocarbons and carbon deposition on Ni-YSZ cermets. J. Power Sources 2002, 112, 588–595. [Google Scholar] [CrossRef]
- Ringuedé, A.; Fagg, D.P.; Frade, J.R. Electrochemical behavior and degradation of (Ni,M)/YSZ cermet electrodes (M = Co,Cu,Fe) for high temperature applications of solid electrolytes. J. Eur. Ceram. Soc. 2004, 24, 1355–1358. [Google Scholar] [CrossRef]
- Shabri, H.A.; Othman, M.H.D.; Mohamed, M.A.; Kurniawan, T.A.; Jamil, S.M. Recent progress in metal-ceramic anode of solid oxide fuel cell for direct hydrocarbon fuel utilization: A review. Fuel Process. Technol. 2021, 212, 106626. [Google Scholar] [CrossRef]
- Rafique, M.; Nawaz, H.; Rafique, M.S.; Tahir, M.B.; Nabi, G.; Khalid, N.R. Material and method selection for efficient solid oxide fuel cell anode: Recent advancements and reviews. Int. J. Energy Res. 2019, 43, 2423–2446. [Google Scholar] [CrossRef]
- Cascos, V.; Martínez-Coronado, R.; Fernández-Díaz, M.T.; Alonso, J.A. Topotactic oxidation of perovskites to novel SrMo1-xMxO4 (M = Fe and Cr) deficient scheelite-type oxides. Materials 2020, 13, 4441. [Google Scholar] [CrossRef]
- Faro, M.L.; Zignani, C.S.; Aricò, A.S. Lanthanum ferrites-based exsolved perovskites as fuel-flexible anode for solid oxide fuel cells. Materials 2020, 13, 3231. [Google Scholar] [CrossRef]
- Das, A.; Kumar, S.; Kumar, S.; Omar, S. High-performance SrFe0.1Mo0.9O3-δ-based composites for the anode application in solid oxide fuel cells. Electrochim. Acta 2020, 354, 136759. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F.; Xia, C. A review on cathode processes and materials for electro-reduction of carbon dioxide in solid oxide electrolysis cells. J. Power Sources 2021, 493, 229713. [Google Scholar] [CrossRef]
- Graves, C.R.; Reddy Sudireddy, B.; Mogensen, M.B. Molybdate based ceramic negative-electrode materials for solid oxide cells. ECS Trans. 2010, 28, 173–192. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Świerczek, K. A- and B-site doping effect on physicochemical properties of Sr2-xBaxMMoO6 (M = Mg, Mn, Fe) double perovskites—Candidate anode materials for SOFCs. Funct. Mater. Lett. 2016, 9, 164–1002. [Google Scholar] [CrossRef]
- Vasala, S.; Lehtimäki, M.; Huang, Y.H.; Yamauchi, H.; Goodenough, J.B.; Karppinen, M. Degree of order and redox balance in B-site ordered double-perovskite oxides, Sr2MMoO6-δ (M = Mg, Mn, Fe, Co, Ni, Zn). J. Solid State Chem. 2010, 183, 1007–1012. [Google Scholar] [CrossRef]
- Liu, Q.; Bugaris, D.E.; Xiao, G.; Chmara, M.; Ma, S.; zur Loye, H.-C.; Chen, F. Sr2Fe1.5Mo0.5O6-δ as a regenerative anode for solid oxide fuel cells. J. Power Sources 2011, 196, 9148–9153. [Google Scholar] [CrossRef]
- Zheng, K.; Swierczek, K. Physicochemical properties of rock salt-type ordered Sr2MMoO6 (M = Mg, Mn, Fe, Co, Ni) double perovskites. J. Eur. Ceram. Soc. 2014, 34, 4273–4284. [Google Scholar] [CrossRef]
- Zheng, К.; Swierczek, K.; Zając, W.; Klimkowicz, A. Rock salt ordered-type double perovskite anode materials for solid oxide fuel cells. Solid State Ion. 2014, 257, 9–16. [Google Scholar] [CrossRef]
- Huang, Y.H.; Liang, G.; Croft, M.; Lehtimaki, M.; Karppinen, M.; Goodenough, J.B. Double-perovskite anode materials Sr2MMoO6 (M = Co, Ni) for solid oxide fuel cells. Chem. Mater. 2009, 21, 2319–2326. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Du, Z.; Chen, T.; Chen, N. Electrical, chemical and electrochemical properties of double perovskite oxides Sr2Mg1−xNixMoO6-δ as anode materials for solid oxide fuel cells. J. Phys. Chem. C 2014, 118, 18853–18860. [Google Scholar] [CrossRef]
- Afroze, S.; Karim, A.H.; Cheok, Q.; Eriksson, S.; Azad, A.K. Latest development of double perovskite electrode materials for solid oxide fuel cells: A review. Front. Energy 2019, 13, 770–797. [Google Scholar] [CrossRef]
- Feng, T.A.B.; Niu, B.C.; Liu, J.A.; He, T.A. Sr- and Mo-deficiency Sr1.95TiMo1−xO6-δ double perovskites as anodes for solid-oxide fuel cells using H2S-containing syngas. Int. J. Hydrog. Energy 2020, 45, 23444–23454. [Google Scholar] [CrossRef]
- Peng, X.A.B.; Tian, Y.A.; Liu, Y.B.; Wang, W.A.; Jing, C.; Li, J.D.; Chi, B.A.; Pu, J.A.; Li, J.A. A double perovskite decorated carbon-tolerant redox electrode for symmetrical SOFC. Int. J. Hydrog. Energy 2020, 45, 14461–14469. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Liu, J.; Zhang, Y.; Jiang, P.; Lee, T.; Xu, B.; He, T. Highly carbon– and sulfur–tolerant Sr2TiMoO6-δ double perovskite anode for solid oxide fuel cells. Int. J. Hydrog. Energy 2019, 44, 20404–20415. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, Y.H.; Cheng, J.G.; Mao, Z.Q.; Goodenough, J.B. Sr2CoMoO6 anode for solid oxide fuel cell running on H2 and CH4 fuels. J. Power Sources 2011, 196, 1738–1743. [Google Scholar] [CrossRef]
- Chroneos, A.; Yildiz, B.; Tarancón, A.; Parfitt, D.; Kilner, J.A. Oxygen diffusion in solid oxide fuel cell cathode and electrolyte materials: Mechanistic insights from atomistic simulations. Energy Environ. Sci. 2011, 4, 2774–2789. [Google Scholar] [CrossRef]
- Kharton, V.V.; Shaula, A.L.; Vyshatko, N.P.; Marques, F.M.B. Electron–hole transport in (La0.9Sr0.1)0.98Ga0.8Mg0.2O3-δ electrolyte: Effects of ceramic microstructure. Electrochim. Acta 2003, 48, 1817–1828. [Google Scholar] [CrossRef]
- Morales, M.; Roa, J.J.; Tartaj, J.; Segarra, M. A review of doped lanthanum gallates as electrolytes for intermediate temperature solid oxides fuel cells: From materials processing to electrical and thermo-mechanical properties. J. Eur. Ceram. Soc. 2016, 36, 1–16. [Google Scholar] [CrossRef]
- Gwan, M.A.; Yun, J.W. Carbon tolerance effects of Sr2NiMoO6-δ as an alternative anode in solid oxide fuel cell under methane fuel condition. J. Electroceram. 2018, 40, 171–179. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Xu, C.; Liu, Y.; He, B.; Chen, C. Double perovskite oxides Sr2Mg1-xFexMoO6-δ for catalytic oxidation of methane. Nat. Gas Chem. 2011, 156, 345–349. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Zhao, N.; Liu, Y.; He, B.; Hu, F.; Chen, C. Structure properties and catalytic performance in methane combustion of double perovskites Sr2Mg1-xMnxMoO6-δ. Appl. Catal. B 2010, 102, 78–84. [Google Scholar] [CrossRef]
- Howell, T.; Kuhnell, C.; Reitz, T. A2MgMoO6 (A = Sr, Ba) for use as sulfur tolerant anodes. J. Power Sources 2013, 231, 279–284. [Google Scholar] [CrossRef]
- Zheng, K.; Świerczek, K.; Carcases, N.M.; Norby, T. Coking study in anode materials for SOFCs: Physicochemical properties and behavior of Mo-containing perovskites in CO and CH4 fuels. J. Electrochem. Soc. 2014, 64, 103–116. [Google Scholar] [CrossRef]
- Tan, W.; Pan, C.; Yang, S.; Zhong, Q. Application of symmetric solid oxide fuel cell in fuel containing sulfur: I. Effect of electrodes. J. Power Sources 2015, 277, 416–425. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Y.; Li, Y. Direct CH4 fuel cell using Sr2FeMoO6 as an anode material. J. Power Sources 2011, 196, 6104–6109. [Google Scholar] [CrossRef]
- Escudero, M.; Gómez de Parada, I.; Fuerte, A.; Daza, L. Study of Sr2Mg(Mo0.8Nb0.2)O6-δ as anode material for solid oxide fuel cells using hydrocarbons as fuel. J. Power Sources 2013, 243, 654–660. [Google Scholar] [CrossRef]
- Falcon, H.; Barbero, J.A.; Araujo, G.; Casais, M.T.; Martı́nez-Lope, M.J.; Alonso, J.A.; Fierro, J.L. Double perovskite oxides A2FeMoO6-δ (A = Ca, Sr and Ba) as catalysts for methane combustion. J. Appl. Catal. B 2004, 53, 37–45. [Google Scholar] [CrossRef]
- Goodenough, J.; Huang, Y.H. Alternative anode materials for solid oxide fuel cells. J. Power Sources 2007, 173, 1–10. [Google Scholar] [CrossRef]
- Ji, Y.; Huang, Y.-H.; Ying, J.R.; Goodenough, J.B. Electrochemical performance of La-doped Sr2MgMoO6-δ in natural gas. Electrochem. Commun. 2007, 9, 1881–1885. [Google Scholar] [CrossRef]
- Wang, F.Y.; Zhong, G.B.; Luo, S.; Xia, L.; Fang, L.-H.; Song, X.; Hao, X.; Yan, G. Porous Sr2MgMo1-xVxO6 ceramics as anode materials for SOFCs using biogas fuel. Catal. Commun. 2015, 67, 108–111. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Xiao, G.; Yang, Z.; Han, M.; Chen, F. Sulfur-tolerant hierarchically porous ceramic anode-supported solid-oxide fuel cells with self-precipitated nanocatalyst. ChemElectroChem 2015, 2, 672–678. [Google Scholar] [CrossRef]
- Chang, H.; Chen, H.; Yang, G.; Shi, J.; Zhou, W.; Bai, J.; Li, S.-D. Enhanced coking resistance of Ni cermet anodes for solid oxide fuel cells based on methane on-cell reforming by a redox-stable double-perovskite Sr2MoFeO6-δ. Int. J. Energ. Res. 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Li, G.; Gou, Y.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Recent progress of tubular solid oxide fuel cell: From materials to applications. J. Power Sources 2020, 477, 228693. [Google Scholar] [CrossRef]
- Sobyanin, V.A. High-temperature solid oxide fuel cells and methane conversion. Ross. Khim. Zh. 2003, 47, 62–70. (In Russian) [Google Scholar]
- Kawada, T.; Mizusaki, J. Current electrolytes and catalysts. In Handbook of Fuel Cells-Fundamentals, Technology and Application; Vielstich, W., Lamm, A., Gasteiger, A., Eds.; Wiley and Sons: Chichester, UK, 2003; Volume 4, pp. 987–1001. [Google Scholar]
- Ramadhani, F.; Hussain, M.A.; Mokhlis, H.; Hajimolana, S. Optimization strategies for solid oxide fuel cell (SOFC) application: A literature survey. Renew. Sust. Energ. Rev. 2017, 76, 460–484. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Thattai, A.T.; Fan, L.; Lindeboom, R.E.F.; Spanjers, H.; Aravind, P.V. Solid oxide fuel cells fuelled with biogas: Potential and constraints. J. Renew Energ. 2019, 134, 194–214. [Google Scholar] [CrossRef]
- Shi, N.; Xie, Y.; Yang, Y.; Xue, S.; Li, X.; Zhu, K.; Huan, D.; Peng, R.; Xia, C.; Lu, Y. Review of anodic reactions in hydrocarbon fueled solid oxide fuel cells and strategies to improve anode performance and stability. Mater. Renew. Sustain. Energy 2020, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Veluswamy, G.K.; Laycock, C.J.; Shah, K.; Ball, A.S.; Guwy, A.J.; Dinsdale, R.M. Biohythane as an energy feedstock for solid oxide fuel cells. Int. J. Hydrog. Energy 2019, 44, 27896–27906. [Google Scholar] [CrossRef]
- Thiruselvi, D.A.; Kumar, P.S.B.; Kumar, M.A.C.; Lay, C.H.; Aathika, S.A.; Mani, Y.A.; Jagadiswary, D.G.; Dhanasekaran, A.H.; Shanmugam, P.I.; Sivanesan, S.A.; et al. A critical review on global trends in biogas scenario with its up-gradation techniques for fuel cell and future perspectives. Int. J. Hydrog. Energy 2021. [Google Scholar] [CrossRef]
- Yu, F.; Han, T.; Wang, Z.; Xie, Y.; Wu, Y.; Jin, Y.; Yang, N.; Xiao, J.; Kawi, S. Recent progress in direct carbon solid oxide fuel cell: Advanced anode catalysts, diversified carbon fuels, and heat management. Int. J. Hydrog. Energy 2021, 46, 4283–4300. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Munnings, C.; Kulkarni, A. Review of progress in high temperature solid oxide fuel cells. J. Aust. Ceram. Soc. 2014, 50, 23–37. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Uppadhya, S.; Parkash, O. A brief review on ceria based solid electrolytes for solid oxide fuel cells. J. Alloys Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Kawashima, T.; Miyoshi, S.; Shibuta, Y.; Yamaguchi, S. Particle size dependence of polarization of Ni/YSZ cermet anodes for solid oxide fuel cells. J. Power Sources 2013, 234, 147–153. [Google Scholar] [CrossRef]
- Barnett, S.A.; Park, B.-K.; Scipioni, R. Effect of infiltration on performance of Ni-YSZ fuel electrodes. ECS Transact. 2019, 91, 1791–1797. [Google Scholar] [CrossRef]
- Rösch, B.; Tu, H.; Störmer, A.O.; Müller, A.C.; Stimming, U. Electrochemical characterization of Ni-Ce0.9Gd0.1O2-δ for SOFC anodes. Solid State Ion. 2004, 175, 113–117. [Google Scholar] [CrossRef]
- Siang, T.J.; Jalil, A.A.; Hambali, H.U.; Hussain, I.; Azami, M.S. Catalytic partial oxidation of methane to syngas over perovskite catalysts. E3S Web Conf. 2019, 90, 01006. [Google Scholar] [CrossRef]
- Shu, L.; Sunarso, J.; Hashim, S.S.; Mao, J.; Zhou, W.; Liang, F. Advanced perovskite anodes for solid oxide fuel cells: A review. Int. J. Hydrog. Energy 2019, 44, 31275–31304. [Google Scholar] [CrossRef]
- Alvarado Flores, J.J.; Ávalos Rodríguez, M.L.; Andrade Espinosa, G.; Alcaraz Vera, J.V. Advances in the development of titanates for anodes in SOFC. Int. J. Hydrog. Energy 2019, 44, 12529–12542. [Google Scholar] [CrossRef]
- Zhou, X.W.; Yan, N.; Chuang, K.T.; Luo, J.L. Progress in La-doped SrTiO3 (LST)-based anode materials for solid oxide fuel cells. RSC Adv. 2014, 4, 118–131. [Google Scholar] [CrossRef] [Green Version]
- Channu, V.S.R.; Holze, R.; Walker, E.H.; Kalluru, R.R. Synthesis and characterization of La0.75Sr0.25Cr0.5Mn0.5O3-δ nanoparticles using a combustion method for solid oxide fuel cells. New J. Glass Ceram. 2011, 1, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Royer, S.; Alamdari, H.; Duprez, D.; Kaliaguine, S. Oxygen storage capacity of La1−xA′xBO3 perovskites (with A′ = Sr, Ce; B = Co, Mn)—relation with catalytic activity in the CH4 oxidation reaction. Appl. Catal. B 2005, 58, 273–288. [Google Scholar] [CrossRef]
- Li, J.; Wei, B.; Cao, Z.; Yue, X.; Zhang, Y.; Lü, Z. Niobium doped lanthanum strontium ferrite as a redox-stable and sulfur-tolerant anode for solid oxide fuel cells. ChemSusChem 2017, 11, 254–263. [Google Scholar] [CrossRef]
- Cao, Z.; Fan, L.; Zhang, G.; Shao, K.; He, C.; Zhang, Q.; Zhu, B. Titanium-substituted ferrite perovskite: An excellent sulfur and coking tolerant anode catalyst for SOFCs. Catal. Today 2019, 330, 217–221. [Google Scholar] [CrossRef]
- Cheng, Z.; Zha, S.; Aguilar, L.; Wang, D.; Winnick, J.; Liu, M. A solid oxide fuel cell running on H2S/CH4 fuel mixtures. Electrochem. Solid State Lett. 2006, 9, A31–A33. [Google Scholar] [CrossRef]
- Eriksson, A.K.; Eriksson, S.-G.; Ivanov, S.A.; Knee, C.S.; Eriksen, J.; Rundlöf, H.; Tseggai, M. High temperature phase transition of the magnetoelectric double perovskite Sr2NiMoO6 by neutron diffraction. Mat. Res. Bull. 2006, 41, 144–157. [Google Scholar] [CrossRef]
- Troncoso, L.; Martínez-Lope, M.J.; Alonso, J.A.; Fernández-Díaz, M.T. Evaluation of Sr2MMoO6 (M = Mg, Mn) as anode materials in solid-oxide fuel cells: A neutron diffraction study. J. Appl. Phys. 2013, 113, 023511. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Serrate, D.; Teresa, J.M.D.; Ibarra, M.R. Double perovskites with ferromagnetism above room temperature. J. Phys. Condens. Matter 2006, 19, 02320. [Google Scholar] [CrossRef]
- Wei, T.; Ji, Y.; Meng, X.; Zhang, Y. Sr2NiMoO6-δ as anode material for LaGaO3-based solid oxide fuel cell. J. Electrochem. Commun. 2008, 10, 1369–1372. [Google Scholar] [CrossRef]
- Jiang, L.; Liang, G.; Han, J.; Huang, Y. Effects of Sr-site deficiency on structure and electrochemical performance in Sr2MgMoO6 for solid-oxide fuel cell. J. Power Sources 2014, 270, 441–448. [Google Scholar] [CrossRef]
- Huang, Y. –H.; Dass, R.J.; Deniszyn, J.C.; Goodenough, J.B. Synthesis and characterization of Sr2MgMoO6-δ an anode material for the solid oxide fuel cell. J. Electrochem. Soc. 2006, 153, 1266–1272. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; He, Q.; He, T. Double-perovskites A2FeMoO6-δ (A = Ca, Sr, Ba) as anodes for solid oxide fuel cells. J. Power Sources 2010, 159, 6356–6366. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Wang, Z.; Qie, F.; Li, Y. An all perovskite direct methanol solid oxide fuel cell with high resistance to carbon formation at the anode. RSC Adv. 2012, 2, 3857. [Google Scholar] [CrossRef]
- Gou, M.; Ren, R.; Sun, W.; Xu, C.; Meng, X.; Wang, Z.; Sun, K. Nb-doped Sr2Fe1.5Mo0.5O6-δ electrode with enhanced stability and electrochemical performance for symmetrical solid oxide fuel cells. Ceram. Int. 2019, 45, 15696–15704. [Google Scholar] [CrossRef]
- Wang, F.; Wang, W.; Ran, R.; Tade, M.O.; Shao, Z. Aluminum oxide as a dual-functional modifier of Ni-based anodes of solid oxide fuel cells for operation on simulated biogas. J. Power Sources 2014, 268, 787–793. [Google Scholar] [CrossRef]
- Ma, M.; Yang, X.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Progress and challenges of carbon-fueled solid oxide fuel cells anode. J. Energy Chem. 2021, 56, 209–222. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, G.; Dai, R.; Lv, X.; Yang, D.; Geng, S. A review of the chemical compatibility between oxide electrodes and electrolytes in solid oxide fuel cells. J. Power Sources 2021, 492, 229630. [Google Scholar] [CrossRef]
- Prasatkhetragarn, A.; Kaowphong, S.; Yimnirun, R. Synthesis, structural and electrical properties of double perovskite Sr2NiMoO6 ceramics. Appl. Phys. A 2012, 107, 117–121. [Google Scholar] [CrossRef]
- Sereda, V.V.; Tsvetkov, D.S.; Sednev, A.L.; Druzhinina, A.I.; Malyshkin, D.A.; Zuev, A.Y. Thermodynamics of Sr2NiMoO6 and Sr2CoMoO6 and their stability under reducing conditions. Phys. Chem. Chem. Phys. 2018, 20, 20108–20116. [Google Scholar] [CrossRef]
- Gagulin, V.V.; Korchagina, S.K.; Ivanova, V.V.; Shevchuk, Y.A. Synthesis and properties of Sr2CoMoO6 and Sr2NiMoO6. Inorg. Mater. 2003, 39, 625–626. [Google Scholar] [CrossRef]
- Eriksson, A.K.; Eriksson, S.G.; Ivanov, S.A. Phase transitions of the magnetoelectric A2NiMoO6 (A = Ba, Sr) and Ca2NiWO6 by neutron diffraction. Ferroelectrics 2014, 339, 235–243. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, R.; Singh, P. Structural and electrical behavior of double perovskite material Sr2NiMoO6. Adv. Sci. Lett. 2014, 20, 647–649. [Google Scholar] [CrossRef]
- Bijelić, J.; Tatar, D.; Hajra, S.; Sahu, M.; Kim, S.J.; Jagličić, Z.; Djerdj, I. Nanocrystalline antiferromagnetic high-κ dielectric Sr2NiMO6 (M = Te, W) with double perovskite structure type. Molecules 2020, 25, 3996. [Google Scholar] [CrossRef]
- Kumar, P.; Presto, S.; Sinha, A.S.K.; Varma, S.; Viviani, M.; Singh, P. Effect of samarium (Sm3+) doping on structure and electrical conductivity of double perovskite Sr2NiMoO6 as anode material for SOFC. J. Alloys Compd. 2017, 725, 1123–1129. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; León-Reina, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Chemical stability and compatibility of double perovskite anode materials for SOFCs. Solid State Ion. 2013, 239, 1–7. [Google Scholar] [CrossRef]
- Filonova, E.A.; Dmitriev, A.S. Physicochemical properties of potential cathode La1-xBaxMn1-yCryO3 and anode Sr2NiMoO6 materials for solid-oxide fuel cells. Euras. Chem. Tech. J. 2012, 14, 139–145. [Google Scholar] [CrossRef]
- He, B.; Wang, Z.; Zhao, L.; Pan, X.; Wu, X.; Xia, C. Ti-doped molybdenum-based perovskites as anodes for solid oxide fuel cells. J. Power Sources 2013, 241, 627–633. [Google Scholar] [CrossRef]
- Tietz, F. Thermal expansion of SOFC materials. Ionics 1999, 5, 129–139. [Google Scholar] [CrossRef]
- Tsipis, E.V.; Kharton, V.V.; Frade, J.R. Electrochemical behavior of mixed–conducting oxide cathodes in contact with apatite–type La10Si5AlO26.5 electrolyte. Electrochim. Acta 2007, 52, 4428–4435. [Google Scholar] [CrossRef]
- Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Gabás, M.; Núñez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Redox behaviour, chemical compatibility and electrochemical performance of Sr2MgMoO6-δ as SOFC anode. Solid State Ion. 2010, 180, 1672–1682. [Google Scholar] [CrossRef]
- Sasaki, K.; Shin-Mura, K. Electrical conductivity of Sr2MgMoO6 for solid oxide fuel cell anodes. J. Ceram. Soc. Jpn. 2017, 125, 487–493. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hara, K.; Onozawa, T.; Shin-Mura, K.; Otani, Y.; Ogawa, S.; Niwa, E.; Hashimoto, T.; Sasaki, K. Synthesis of Sr2MgMoO6 by atmosphere-controlled calcination method and characterization for solid oxide fuel cells. In Advances in Solid Oxide Fuel Cells and Electronic Ceramics II: Ceramic Engineering and Science Proceedings XXXVII; Kusnezoff, M., Bansal, N.P., Shimamura, K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; Volume 37. [Google Scholar] [CrossRef]
- Takano, S.; Shin-Mura, K.; Niwa, E.; Hashimoto, T.; Sasaki, K. Chemical compatibility of Sr2MgMoO6 with representative electrolyte materials and interlayer materials for solid oxide fuel cells. J. Ceram. Soc. Jpn. 2018, 126, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Thomas, T.; Qi, H.; Liua, X.; Zondlo, J.; Sabolsky, E.M.; Hart, R. Effect of Mg/Mo rRatio in a stoichiometric Sr2MgMoO6-δ (SMM) redox-stable anode. J. Electrochem. Soc. 2017, 78, 1205–1215. [Google Scholar] [CrossRef]
- Bernuy–Lopez, C.; Allix, M.; Bridges, C.A.; Claridge, J.B.; Rosseinsky, M.J. Sr2MgMoO6: Structure, phase stability and cation site order control of reduction. Chem. Mater. 2007, 19, 1035–1043. [Google Scholar] [CrossRef]
- Vasala, S.; Yamauchi, H.; Karppinen, M. Role of SrMoO4 in Sr2MgMoO6 synthesis. J. Solid State Chem. 2011, 184, 1312–1315. [Google Scholar] [CrossRef]
- Vasala, S.; Lehtimaki, S.; Haw, S.W.; Chen, J.M.; Liu, R.S.; Yamauchi, H.; Karppinen, M. Isovalent and aliovalent substitution effects on redox chemistry Sr2MgMoO6-δ SOFC-anode material. Solid State Ion. 2010, 181, 754–759. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Du, Z.; Chen, T.; Chen, N.; Liu, X.; Skinner, S.J. Effects of Co doping on the electrochemical performance of double perovskite oxide Sr2MgMoO6-δ as an anode material for solid oxide fuel cells. J. Phys. Chem. 2012, 36, 9734–9743. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Chen, T.; Zhou, X.; Du, Z. Synthesis and electrical properties of Al-doped Sr2MgMoO6 as an anode material for solid oxide fuel cells. Int. J. Hydrog. Energy 2011, 36, 7257–7264. [Google Scholar] [CrossRef]
- Zhang, L.; He, T. Performance of double-perovskite Sr2-xSmxMgMoO6-δ as solid-oxide fuel-cell anodes. J. Power Sources 2011, 196, 8352–8359. [Google Scholar] [CrossRef]
- Dorai, A.K.; Masuda, Y.; Joo, J.H.; Woo, S.K.; Kim, S.D. Influence of Fe doping on the electrical properties of Sr2MgMoO6. Mater. Chem. Phys. 2013, 139, 360–363. [Google Scholar] [CrossRef]
- Marrero-López, D.; Penã-Martínez, J.; Ruiz-Morales, J.C. High temperature phase transition in SOFC anodes based on Sr2MgMoO6-δ. J. Solid State Chem. 2009, 182, 1027–1034. [Google Scholar] [CrossRef]
- Matsuda, Y.; Karppinen, M.; Yamazaki, Y.; Yamauchi, H. Oxygen-vacancy concentration in A2MgMoO6-δ double-perovskite oxides. J. Solid State Chem. 2009, 182, 279–284. [Google Scholar] [CrossRef]
- Montenegro-Hernández, A.; Dager, P.; Caneiro, A.; Mogni, L. Structural properties and electrical conductivity of perovskite type oxides in SOFCs. J. Phys. Conf. Ser. 2019, 1219, 012001. [Google Scholar] [CrossRef]
- Dager, P.K.; Chanquía, C.M.; Mogni, L.; Caneiro, A. Synthesis of pure-phase Sr2MgMoO6 nanostructured powder by the combustion method. Mater. Lett. 2015, 141, 248–251. [Google Scholar] [CrossRef]
- Marrero-López, D.; Penã-Martínez, J.; Ruiz-Morales, J.C.; Pérez-Coll, D.; Aranda, M.A.G.; Núñez, P. Synthesis, phase stability and electrical conductivity of Sr2MgMoO6 anode. Mat. Res. Bull. 2008, 43, 2441–2450. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Zabolotskaya, E.V.; Kellerman, D.G.; Suntsov, A.Y. The physical properties and electrochemical performance of Ca-doped Sr2MgMoO6-δ as perspective anode for solid oxide fuel cells. J. Solid State Electrochem. 2018, 22, 1209–1215. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Dass, R.I.; Xing, Z.-L.; Goodenough, J.B. Double perovskites as anode materials for solid-oxide fuel cells. Science 2006, 312, 254–257. [Google Scholar] [CrossRef]
- Rath, M.K.; Lee, K.-T. Superior electrochemical performance of non-precious Co-Ni-Mo alloy catalyst-impregnated Sr2FeMoO6 as an electrode material for symmetric solid oxide fuel cells. Electrochim. Acta 2016, 212, 678–685. [Google Scholar] [CrossRef]
- Rager, J.; Zipperle, M.; Sharma, A.; MacManus-Driscoll, J.L. Oxygen stoichiometry in Sr2FeMoO6, the determination of Fe and Mo valence states, and the chemical phase diagram of SrO–Fe3O4–MoO3. J. Am. Ceram. Soc. 2004, 87, 1330–1335. [Google Scholar] [CrossRef]
- Farzin, Y.A.; Babaei, A.; Ataie, A. Low-temperature synthesis of Sr2FeMoO6 double perovskite; structure, morphology, and magnetic properties. Ceram. Int. 2020, 46, 16867–16878. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Steparuk, A.S.; Zuev, A.Y. The defect structure and chemical lattice strain of the double perovskites Sr2BMoO6-δ (B = Mg, Fe). Dalton Trans. 2016, 45, 12906–12913. [Google Scholar] [CrossRef]
- Kircheisen, R.; Töpfer, J. Nonstoichiometry, point defects and magnetic properties in Sr2FeMoO6-δ double perovskites. J. Solid State Chem. 2012, 185, 76–81. [Google Scholar] [CrossRef]
- Sugahara, T.; Ohtaki, M.; Souma, T. Thermoelectric properties of double-perovskite oxides Sr2-xMxFeMoO6 (M = Ba, La). J. Ceram. Soc. Jpn. 2008, 116, 1278–1282. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-García, A.B.; Pavone, M.; Carter, E.A. Effect of antisite defects on the formation of oxygen vacancies in Sr2FeMoO6: Implications for ion and electron transport. Chem. Mater. 2011, 23, 4525–4536. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Zhao, F.; Zhang, L.; Xi, C.; Chen, F. Sr2Fe1.5Mo0.5O6 as cathodes for intermediate-temperature solid oxide fuel cells with La0.8Sr0.2Ga0.87Mg0.13O3 electrolyte. J. Electrochem. Soc. 2011, 158, B455. [Google Scholar] [CrossRef] [Green Version]
- Dai, N.; Feng, J.; Wang, Z.; Jiang, T.; Sun, W.; Qiao, J.; Sun, K. Synthesis and characterization of B-site Ni-doped perovskites Sr2Fe1.5-xNixMo0.5O6 (x = 0, 0.05, 0.1, 0.2, 0.4) as cathodes for SOFCs. J. Mater. Chem. A 2013, 1, 14147–14153. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, W.; Wang, W.; Wang, Z.; Sun, W.; Zhang, J.; Sun, K. The Ca element effect on the enhancement performance of Sr2Fe1.5Mo0.5O6-δ perovskite as cathode for intermediate-temperature solid oxide fuel cells. J. Power Sources 2016, 331, 400–407. [Google Scholar] [CrossRef]
- Feng, J.; Yang, G.; Dai, N.; Wang, Z.; Sun, W.; Rooney, D.; Qiao, J.; Sun, K. Investigation into the effect of Fe-site substitution on the performance of Sr2Fe1.5Mo0.5O6 anodes for SOFCs. J. Mater. Chem. 2014, 2, 17628–17634. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Z.; He, B.; Wang, S.; Wu, X.; Xi, C. Effect of Co doping on the electrochemical properties of Sr2Fe1.5Mo0.5O6 electrode for solid oxide fuel cell. Int. J. Hydrog. Energy 2013, 38, 4108–4115. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Nuansaeng, S.; Chen, F. Sr2Fe1+xMo1-xO6-δ as anode materials for solid oxide fuel cells. ECS Trans. 2012, 45, 355–362. [Google Scholar] [CrossRef]
- Wright, J.H.; Virkar, A.V.; Liu, Q.; Chen, F. Electrical characterization and water sensitivity of Sr2Fe1.5Mo0.5O6-δ as a possible solid oxide fuel cell electrode. J. Power Sources 2013, 237, 13–18. [Google Scholar] [CrossRef]
- Yang, G.; Feng, J.; Sun, W.; Dai, N.; Hou, M.; Hao, X.; Sun, K. The characteristic of strontium-site deficient perovskites SrxFe1.5Mo0.5O6-δ (x = 1.9–2.0) as intermediate-temperature solid oxide fuel cell cathodes. J. Power Sources 2014, 268, 771–777. [Google Scholar] [CrossRef]
- Qi, H.; Lee, Y.-L.; Yang, T.; Li, W.; Li, W.; Ma, L.; Hu, S.; Duan, Y.; Hackett, G.A.; Liu, X. Positive effects of H2O on the hydrogen oxidation reaction on Sr2Fe1.5Mo0.5O6-δ-based perovskite anodes for solid oxide fuel cells. ACS Catal. 2020, 10, 5567–5578. [Google Scholar] [CrossRef]
- Qiu, P.; Lin, J.; Lei, L.; Yuan, Z.; Jia, L.; Li, J.; Chen, F. Evaluation of Cr-tolerance of Sr2Fe1.5Mo0.5O6-δ cathode for solid oxide fuel cells. ACS Appl. Energy Mater. 2019, 2, 7619–7627. [Google Scholar] [CrossRef]
- Osinkin, D.A. Kinetics of CO oxidation and redox cycling of Sr2Fe1.5Mo0.5O6-δ electrode for symmetrical solid state electrochemical devices. J. Power Sources 2019, 418, 17–23. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Li, H.; Zhao, Y.; Li, Y. Synthesis and enhanced electrochemical performance of Sm-doped Sr2Fe1.5Mo0.5O6. Fuel Cells 2014, 14, 973–978. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Kolchugin, A.A.; Bogdanovich, N.M.; Beresnev, S.M. Performance and redox stability of a double–layer Sr2Fe1.5Mo0.5O6-δ-based electrode for solid state electrochemical application. Electrochim. Acta 2020, 361, 137058. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Xiao, G.; Zhao, F.; Chen, F. A novel electrode material for symmetrical SOFCs. Adv. Mater. 2010, 22, 5478–5482. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Wang, Y.; Li, Y. Sr2Fe2−xMoxO6-δ perovskite as an anode in a solid oxide fuel cell: Effect of the substitution ratio. Catal. Today 2016, 259, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Bugaris, D.E.; Hodges, J.P.; Huq, A.; Chance, W.M.; Heyden, A.; Chen, F.; zur Loye, H.-C. Investigation of the high-temperature redox chemistry of Sr2Fe1.5Mo0.5O6-δ via in situ neutron diffraction. J. Mater. Chem. A 2014, 2, 4045–4054. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.; Ammal, S.C.; Suthirakun, S.; Chen, F.; Terejanu, G.A.; Heyden, A. Mechanism of sulfur poisoning of Sr2Fe1.5Mo0.5O6-δ perovskite anode under solid oxide fuel cell conditions. J. Phys. Chem. C 2014, 118, 23545–23552. [Google Scholar] [CrossRef]
- Presto, S.; Kumar, P.; Varma, S.; Viviani, M.; Singh, P. Electrical conductivity of NiMo–based double perovskites under SOFC anodic conditions. Int. J. Hydrog. Energy 2018, 43, 4528–4533. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, N.K.; Sinha, A.S.K.; Singh, P. Structural and electrical characterizations of cerium (Ce3+)-doped double perovskite system Sr2NiMoO6-δ. Appl. Phys. A 2016, 122, 828. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, N.K.; Gupta, G.; Singh, P. Effect of lanthanum (La3+) doping on the structural and electrical properties of double perovskite Sr2NiMoO6. RSC Adv. 2016, 6, 22094–22102. [Google Scholar] [CrossRef]
- Filonova, E.A.; Skutina, L.S.; Medvedev, D.A. Phase transitions and thermal expansion of Sr2-xBaxNiMoO6 and Sr2Ni1-yZnyMoO6 solid solutions. Inorg. Mat. 2016, 52, 57–62. [Google Scholar] [CrossRef]
- Filonova, E.A.; Gilev, A.R.; Skutina, L.S.; Vylkov, A.I.; Kuznetsov, D.K.; Shur, V.Y. Double Sr2Ni1-xMgxMoO6 perovskites (x = 0, 0.25) as perspective anode materials for LaGaO3-based solid oxide fuel cells. Solid State Ion. 2018, 314, 112–118. [Google Scholar] [CrossRef]
- Filonova, E.A.; Dmitriev, A.S.; Pikalov, P.S.; Medvedev, D.A.; Pikalova, E.Y. The structural and electrical properties of Sr2Ni0.75Mg0.25MoO6 and its compatibility with solid state electrolytes. Solid State Ion. 2014, 262, 365–369. [Google Scholar] [CrossRef]
- Skutina, L.S.; Vylkov, A.I.; Grzhegorzhevskii, K.V.; Chuikin, A.Y.; Ostroushko, A.A.; Filonova, E.A. Crystal structure and phase transitions of Sr2Ni1-yMgyMoO6 solid solutions. Inorg. Mat. 2017, 53, 1293–1299. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Cherepanov, V.A. Performance of the lanthanum gallate based solid oxide fuel cells with the La2−xCaxNi1−yFeyO4+δ cathodes and Sr2Ni0.75Mg0.25MoO6-δ anode. Solid State Ion. 2019, 339, 115001. [Google Scholar] [CrossRef]
- Skutina, L.S.; Vylkov, A.A.; Kuznetsov, D.K.; Medvedev, D.A.; Shur, V.Y. Tailoring Ni and Sr2Mg0.25Ni0.75MoO6-δ cermet compositions for designing the fuel electrodes of solid oxide electrochemical cells. Energies 2019, 12, 2394. [Google Scholar] [CrossRef] [Green Version]
- Filonova, E.A.; Russkikh, O.V.; Skutina, L.S.; Vylkov, A.I.; Maksimchuk, T.Y.; Ostroushko, A.A. Sr2Ni0.7Mg0.3MoO6-δ: Correlation between synthesis conditions and functional properties as anode material for intermediate-temperature SOFCs. Int. J. Hydrog. Energy 2021, in press. [Google Scholar] [CrossRef]
- Skutina, L.S.; Vylkov, A.I.; Medvedev, D.A.; Filonova, E.A. Features of structural, thermal and electrical properties of Mo-based composite materials as fuel electrodes for high-temperature applications. J. Alloys Compd. 2017, 705, 854–861. [Google Scholar] [CrossRef]
- Urusova, N.; Kumar, M.R.; Semkin, M.; Filonova, E.; Kratochvilova, M.; Neznakhin, D.; Grzhegorzhevskii, K.; Ostroushko, A.; Park, J.G.; Pirogov, A. Crystal structure and magnetic properties of Sr2Ni1-xMgxMoO6 (x = 0, 0.25, 0.5, and 0.75) polycrystals. Solid State Sci. 2020, 99, 106008. [Google Scholar] [CrossRef]
- Filonova, E.A.; Russkikh, O.V.; Skutina, L.S.; Kochetova, N.A.; Korona, D.V.; Ostroushko, A.A. Influence of synthesis conditions on phase formation and functional properties of prospective anode material Sr2Ni0.75Mg0.25MoO6-δ. J. Alloys Compd. 2018, 48, 671–678. [Google Scholar] [CrossRef]
- Dager, P.K.; Mogni, L.V.; Zampieri, G.; Tobia, D.; Caneiro, A. Effect of transition metal doping on the crystal structure and oxidation state of Sr2MgMo0.9TM0.1O6-δ compounds (TM = Co, Mn or Ni). J. Phys. Chem. Solids 2019, 135, 109084. [Google Scholar] [CrossRef]
- Dager, P.K.; Mogni, L.V.; Soria, S.; Caneiro, A. High temperature properties of Sr2MgMo0.9TM0.1O6-δ (TM = Mn, Co and Ni). Ceram. Int. 2018, 44, 2539–2546. [Google Scholar] [CrossRef]
- Dager, P.K.; Mogni, L.V.; Zampieri, G.; Caneiro, A. Study of Sr2MgMo0.9Ni0.1O6-δ as SOFC anode. ECS Trans. 2017, 78, 1367–1374. [Google Scholar] [CrossRef]
- Xi, X.; Liu, J.; Fan, F.; Wang, L.; Li, J.; Li, M.; Luo, J.-L.; Fu, X.-Z. Reducing d-p band coupling to enhance CO2 electrocatalytic activity by Mg-doping in Sr2FeMoO6-δ double perovskite. Nano Energy 2021, 82, 105707. [Google Scholar] [CrossRef]
- Rosas, J.L.; León-Flores, J.; Escamilla, R.; Cervantes, J.M.; Carvajal, E.; Verdín, E.; Romero, M. LDA+U study of the electronic and magnetic properties of the Sr2FeMo1-xNbxO6 compound. Mater. Today Commun. 2020, 23, 101155. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, X.; Wang, X.; Wang, S. Effects of Nd doping on the magnetocaloric properties of double perovskite Sr2FeMoO6. J. Mag. Mag. Mater. 2019, 499, 166212. [Google Scholar] [CrossRef]
- Reyes, A.M.; Montoya, J.A.; Navarro, O. Demise of half-metallicity upon increasing of disorder in the double perovskite Sr2-yLayFeMoO6. J. Mag. Mag. Mater. 2020, 495, 165877. [Google Scholar] [CrossRef]
- Kim, S.B.; Hahn, E.J.; Kim, C.S. Crystallographic and magnetic properties of double perovskite oxide Ba2-xSrxFeMoO6. J. Kor. Phys. Soc. 2019, 75, 466–470. [Google Scholar] [CrossRef]
- Tian, C.; Cheng, J.; Yang, J. A highly active cathode material of Cu-doped Sr2Fe1.5Mo0.5O6 for symmetrical solid oxide fuel cells. J. Mater. Sci. Mater. Electron. 2021, 32, 1258–1264. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Y.; Zhao, Y.; Zhang, T.; Yu, N.; Chen, X.; Miao, M.; Liu, T. In-situ exsolution of nanoparticles from Ni substituted Sr2Fe1.5Mo0.5O6 perovskite oxides with different Ni doping contents. Electrochim. Acta 2020, 348, 136351. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, S.; Lin, Y.; Chen, F. Ni-doped Sr2Fe1.5Mo0.5O6 as anode materials for solid oxide fuel cells. ECS Trans. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Xu, C.; Sun, K.; Yang, X.; Ma, M.; Ren, R.; Qiao, J.; Wang, Z.; Zhen, S.; Sun, W. Highly active and CO2-tolerant Sr2Fe1.3Ga0.2Mo0.5O6-δ cathode for intermediate-temperature solid oxide fuel cells. J. Power Sources 2020, 450, 227722. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yang, Z.; Lei, Z.; Jin, C.; Liu, Y.; Wang, Y.; Peng, S. Co-substituted Sr2Fe1.5Mo0.5O6-δ as anode materials for solid oxide fuel cells: Achieving high performance via nanoparticle exsolution. J. Power Sources 2019, 438, 226989. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Xia, C.; Bouwmeester, H.J.M. Sr2Fe1.4Mn0.1Mo0.5O6-δ perovskite cathode for highly efficient CO2 electrolysis. J. Mater. Chem. A 2019, 7, 22939. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K. Ti-doped Sr2Fe1.4-xTixMo0.6O6-δ double perovskites with improved stability as anode materials for solid oxide fuel cells. Mat. Res. Bull. 2020, 128, 110877. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Meng, X.; Wang, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Tailoring the oxygen vacancy to achieve fast intrinsic proton transport in perovskite cathode for protonic ceramic fuel cells. ACS Appl. Energy Mater. 2020, 3, 4914–4922. [Google Scholar] [CrossRef]
- Lv, H.; Lin, L.; Zhang, X.; Song, Y.; Matsumoto, H.; Zeng, C.; Ta, N.; Liu, W.; Gao, D.; Wang, G.; et al. In Situ investigation of reversible exsolution/dissolution of CoFe alloy nanoparticles in a Co-doped Sr2Fe1.5Mo0.5O6-δ cathode for CO2 electrolysis. Adv. Mater. 2020, 32, e1906193. [Google Scholar] [CrossRef]
- Qi, H.; Xia, F.; Yang, T.; Li, W.; Li, W.; Ma, L.; Collins, G.; Shi, W.; Tian, H.; Hu, S.; et al. In Situ exsolved nanoparticles on La0.5Sr1.5Fe1.5Mo0.5O6-δ anode enhance the hydrogen oxidation reaction in SOFCs. J. Electrochem. Soc. 2020, 167, 024510. [Google Scholar] [CrossRef]
- Qi, H.; Yang, T.; Li, W.; Ma, L.; Hu, S.; Shi, W.; Sabolsky, E.M.; Zondlo, J.; Hart, R.; Hackett, G.A.; et al. Reversible in-situ exsolution of Fe catalyst in La0.5Sr1.5Fe1.5Mo0.5O6-δ anode for SOFCs. ECS Trans. 2019, 91, 1701–1710. [Google Scholar] [CrossRef]
- Xia, T.; Meng, X.; Luo, T.; Zhan, Z.-L. Synthesis and evaluation of Ca-doped Sr2Fe1.5Mo0.5O6-δ as symmetrical electrodes for high performance solid oxide fuel cells. J. Inorg. Mater. 2019, 34, 1109–1114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhu, Z.; Gu, Y.; Chen, H.; Zheng, Y.; Ge, L. Effect of Cl doping on the electrochemical performance of Sr2Fe1.5Mo0.5O6-δ cathode material for solid oxide fuel cells. Ceram. Int. 2020, 46, 22787–22796. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, W.; Xu, C.; Ren, R.; Yang, X.; Qiao, J.; Wang, Z.; Sun, K. Attenuating metal-oxygen bond of double perovskite oxide via anion doping to enhance its catalytic activity for oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 14091–14098. [Google Scholar] [CrossRef]
















| Electrolyte Thickness, µm | Cathode Composition | Conditions | Pmax, mW cm−2 | Ref. |
|---|---|---|---|---|
| Sr2NiMoO6-δ | ||||
| 300 | SrCo0.8Fe0.2O3-δ | H2, 800 °C 3% H2O/CH4, 800 °C CH4, 800 °C | 480 110 270 | [24] |
| 300 | Ba0.5Sr0.5Co0.8Fe0.2O3-δ | H2, 850 °C H2, 800 °C H2, 750 °C | 820 595 400 | [75] |
| Sr2MgMoO6-δ | ||||
| 280 | Ba0.5Sr0.5Co0.8Fe0.2O3-δ | H2, 800 °C | 660 | [76] |
| 300 | SrCo0.8Fe0.2O3-δ | H2, 800 °C CH4, 800 °C | 840 440 | [77] |
| 300 | Ba0.5Sr0.5Co0.8Fe0.2O3-δ | H2, 850 °C H2, 800 °C CH4, 850 °C CH4, 800 °C | 860 600 605 430 | [40] |
| 300 | SmBaCo2O5+δ | H2, 850 °C H2, 800 °C H2, 750 °C | 830 585 410 | [78] |
| 300 | Ba0.5Sr0.5Co0.8Fe0.2O3-δ | H2, 800 °C | 520 | [79] |
| 1200 | Sr2Fe1.5Mo0.5O6-δ | 3% Н2О/Н2, 800 °C | 375 | [80] |
| 30 | La0.8Sr0.2MnO3–YSZ | H2, 850 °C | 667 | [81] |
| 30 | La0.8Sr0.2MnO3–YSZ | biogas, 850 °C | 520 | [81] |
| 400 | Sr2MnMoO6-δ/NiO–Ce0.8Sm0.2O2-δ | CH4, 800 °C | 245 | [28] |
| Temperature Range, °C | α·106, K−1 | Ref. |
|---|---|---|
| 27–950 | 12.1 | [75] |
| 30–575 | 12.4 | [92] |
| 575–1100 | 14.0 | [92] |
| 20–1300 | 12.9 | [93] |
| Measuring Conditions | σ, S cm−1 | Ref. |
|---|---|---|
| 5%H2/Ar, 800 °C | 0.1 | [24] |
| H2, 800 °C | 1.1 | [24] |
| CH4, 800 °C | 1.1 | [24] |
| H2, 800 °C | 1.6 | [39] |
| H2, 850 °C | 49 | [75] |
| pO2 = 1 × 10−6 Pa, 600 °C | 7·10−4 | [88] |
| Temperature Range, °C | α·106, K−1 | Ref. |
|---|---|---|
| 109–360 | 11.7 | [77] |
| 360–800 | 12.7 | [77] |
| 25–800 | 15.1 | [100] |
| 50–1300 | 12.9 | [104] |
| n/a | 13.6 | [113] |
| Measuring Conditions | σ, S cm−1 | Ref. |
|---|---|---|
| pO2 = 10−24, 800 °C | 3.5 | [18] |
| 5%H2/Ar, 800 °C | 9.5 | [45] |
| 5%H2/Ar, 800 °C | 4 | [77] |
| H2, 800 °C | 10 | [77] |
| 5%H2/Ar, 800 °C | 0.07 | [97] |
| 5%H2/N2, 800 °C | 50 | [100] |
| 5%H2/Ar, 900 °C | 0.5 | [104] |
| 5%H2/Ar, 800 °C | 1.5 | [113] |
| Measuring Conditions | σ, S cm−1 | Ref. |
|---|---|---|
| H2, 800 °C | 9 | [78] |
| Air, 800°C | 13 | [80] |
| Air, 800°C | 10 | [98] |
| H2, 800 °C | 16 | [125] |
| Air, 800°C | 17 | [126] |
| H2 (3 % H2O), 800°C | 13 | [127] |
| H2, 780 °C | 310 | [135] |
| Air, 780 °C | 550 | [135] |
| H2, 800 °C | 41 | [136] |
| Temperature Range, °C | α·106, K−1 | Ref. |
|---|---|---|
| 200–760 | 14.5 | [122] |
| 760–1200 | 21.4 | [122] |
| 200–1200 | 18.1 | [122] |
| 40–350 | 11.6 | [123] |
| 500–800 | 18.6 | [123] |
| 40–950 | 16.3 | [124] |
| 50–450 | 12.8 | [126] |
| 650–900 | 20.2 | [126] |
| System | Concentration, x | Ref. |
|---|---|---|
| Sr2NiMoO6-δ | ||
| Sr2-xCexNiMoO6-δ | x = 0.01 | [139] |
| Sr2-xCexNiMoO6-δ | 0 ≤ x ≤ 0.05 | [140] |
| Sr2-xSmxNiMoO6-δ | 0 ≤ x ≤ 0.05 | [90] |
| Sr2-xLaxNiMoO6-δ | 0 ≤ x ≤ 0.1 | [141] |
| Sr2-xBaxNiMoO6-δ | 0 ≤ x ≤ 1 | [142] |
| Sr2Ni1-xMgxMoO6-δ | 0 ≤ x ≤ 0.25 | [143,144,145,146,147] |
| Sr2Ni1-xMgxMoO6-δ | x = 0.3 | [148] |
| Sr2Ni1-xMgxMoO6-δ | 0 ≤ x ≤ 0.75 | [149,150,151] |
| Sr2Ni1-xMgxMoO6-δ | 0 ≤ x ≤ 1 | [25] |
| Sr2Ni1-xZnxMoO6-δ | 0 ≤ x ≤ 1 | [142] |
| Sr2MgMoO6-δ | ||
| Sr2MgMo1-xCoxO6-δ | x = 0.1 | [110,152,153] |
| Sr2MgMo1-xMnxO6-δ | x = 0.1 | [110,152,153] |
| Sr2MgMo1-xNixO6-δ | x = 0.1 | [110,152,153,154] |
| Sr2-xCaxMgMoO6-δ | 0 ≤ x ≤ 0.5 | [113] |
| Sr2FeMoO6-δ | ||
| Sr2FeMo1-xMgxO6-δ | x = 1/3 | [155] |
| Sr2FeMo1-xNbxO6-δ | 0 ≤ x ≤ 1 | [156] |
| Sr2-xNdxFeMoO6-δ | 0 ≤ x ≤ 0.05 | [157] |
| Sr2-xLaxFeMoO6-δ | 0 ≤ x ≤ 1 | [120,158] |
| Sr2-xBaxFeMoO6-δ | 0 ≤ x ≤ 2 | [120,159] |
| Sr2Fe1.5Mo0.05O6-δ | ||
| Sr2Fe1.5-xCuxMo0.5O6-δ | 0 ≤ x ≤ 0.3 | [160] |
| Sr2Fe1.5-xNixMo0.5O6-δ | 0 ≤ x ≤ 0.4 | [123,161,162] |
| Sr2Fe1.5-xGaxMo0.5O6-δ | x = 0.2 | [163] |
| Sr2Fe1.5-xCoxMo0.5O6-δ | x = 0.2 | [164] |
| Sr2Fe1.5-xCoxMo0.5O6-δ | 0 ≤ x ≤ 1.0 | [126] |
| Sr2Fe1.5-xNbxMo0.5O6-δ | x = 0.1 | [80] |
| Sr2Fe1.5-xMnxMo0.5O6-δ | x = 0.1 | [165] |
| Sr2Fe1.4-xTixMo0.6O6-δ | 0 ≤ x ≤ 0.1 | [166] |
| Sr2Fe1.5Mo0.5-xZrxO6-δ | x = 0.1 | [167] |
| Sr2Fe1.5–3xMo0.5-xCo4xO6-δ | x = 0.05 | [168] |
| Sr2-xLaxFe1.5Mo0.5O6-δ | x = 0.5 | [169,170] |
| Sr2-xCaxFe1.5Mo0.5O6-δ | 0 ≤ x ≤ 0.6 | [171] |
| Sr2Fe1.5Mo0.5O6-δ-xClx | 0 ≤ x ≤ 0.4 | [172] |
| Sr2Fe1.5Mo0.5O6-δ-xFx | 0 ≤ x ≤ 0.3 | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skutina, L.; Filonova, E.; Medvedev, D.; Maignan, A. Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells. Materials 2021, 14, 1715. https://doi.org/10.3390/ma14071715
Skutina L, Filonova E, Medvedev D, Maignan A. Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells. Materials. 2021; 14(7):1715. https://doi.org/10.3390/ma14071715
Chicago/Turabian StyleSkutina, Lubov, Elena Filonova, Dmitry Medvedev, and Antoine Maignan. 2021. "Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells" Materials 14, no. 7: 1715. https://doi.org/10.3390/ma14071715
APA StyleSkutina, L., Filonova, E., Medvedev, D., & Maignan, A. (2021). Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells. Materials, 14(7), 1715. https://doi.org/10.3390/ma14071715