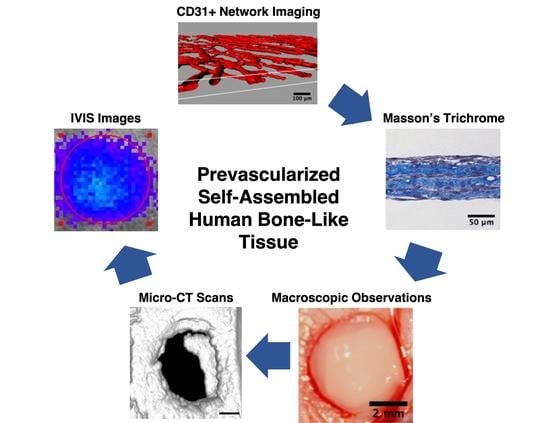
In Vitro Prevascularization of Self-Assembled Human Bone-Like Tissues and Preclinical Assessment Using a Rat Calvarial Bone Defect Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Adipose-Derived Stromal/Stem Cell Isolation, Expansion, and Transduction
2.2. Human Umbilical Vein Endothelial Cell Isolation, Transduction, and Expansion
2.3. Production of Human Prevascularized Bone-Like Substitutes
2.4. Two-Dimensional Characterization of Capillary Network Formation
2.5. Histological Analyses of Substitutes and Explanted Tissues
2.6. Human CD31 Indirect Immunofluorescent Labeling on Substitute’s Cross-Sections
2.7. Transmission Electron Microscopy of Capillary Networks in Substitutes
2.8. CD31 Immunolabeling and 3D Network Reconstruction in Prevascularized Substitutes
2.9. Enzyme-Linked Immunosorbent Assay for Human Osteocalcin in Substitute’s Extracts
2.10. O-Cresolphthalein Complexone Method for Calcium Quantification
2.11. Fluorescent Hydroxyapatite Labeling, Imaging, and Quantification
2.12. Calvarial Bone Defect Surgery and Grafting of the Substitutes
2.13. Micro-Computed Tomography Imaging and Analysis
2.14. Analysis of Graft Survival Using In Vivo Imaging System (IVIS)
2.15. Statistical Analyses
3. Results
3.1. Production of All-Natural and Human Prevascularized Bone-Like Tissues
3.2. Kinetics of Capillary Network Organization on Prevascularized Cell Sheets
3.3. Characterization of Capillary-Like Morphological Features in Prevascularized Tissues
3.4. Three-Dimensional Reconstruction Following Imaging to Evaluate Capillary Network Segments and Volume/Density within the Substitutes
3.5. In Vitro Matrix Deposition of Osteocalcin within the Substitutes
3.6. Matrix Mineralization within the Bone-Like Tissues
3.7. Healing of Calvarial Bone Defects in a Rodent Model
3.8. Micro-Computed Tomography Analysis and Reconstruction of the Grafted Bone Defects
3.9. Human Osteocalcin Content in Tissues 12 Weeks after Implantation
3.10. Viability of Implanted Prevascularized Substitutes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sittitavornwong, S.; Gutta, R. Bone graft harvesting from regional sites. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 317–330. [Google Scholar] [CrossRef]
- Moura, L.B.; Carvalho, P.H.A.; Xavier, C.B.; Post, L.K.; Torriani, M.A.; Santagata, M.; Chagas Júnior, O.L. Autogenous non-vascularized bone graft in segmental mandibular reconstruction: A systematic review. Int. J. Oral Maxillofac. Surg. 2016, 45, 1388–1394. [Google Scholar] [CrossRef]
- Hidalgo, D.A.; Rekow, A. A review of 60 consecutive fibula free flap mandible reconstructions. Plast. Reconstr. Surg. 1995, 96, 585–602. [Google Scholar] [CrossRef]
- Elsalanty, M.E.; Genecov, D.G. Bone grafts in craniofacial surgery. Craniomaxillofac. Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myeroff, C.; Archdeacon, M. Autogenous bone graft: Donor sites and techniques. JBJS 2011, 93, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Lomas, R.J.; Gillan, H.L.; Matthews, J.B.; Ingham, E.; Kearney, J.N. An evaluation of the capacity of differently prepared demineralised bone matrices (DBM) and toxic residuals of ethylene oxide (EtOx) to provoke an inflammatory response in vitro. Biomaterials 2001, 22, 913–921. [Google Scholar] [CrossRef]
- Callan, D.P.; Salkeld, S.L.; Scarborough, N. Histologic analysis of implant sites after grafting with demineralized bone matrix putty and sheets. Implant Dent. 2000, 9, 36–44. [Google Scholar] [CrossRef]
- Wiltfang, J.; Zernial, O.; Behrens, E.; Schlegel, A.; Warnke, P.H.; Becker, S.T. Regenerative treatment of peri-implantitis bone defects with a combination of autologous bone and a demineralized xenogenic bone graft: A series of 36 defects. Clin. Implant Dent. Relat. Res. 2012, 14, 421–427. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yun, P.Y.; Lee, H.J.; Ahn, J.; Kim, S.G. Ridge preservation of the molar extraction socket using collagen sponge and xenogeneic bone grafts. Implant Dent. 2011, 20, 267–272. [Google Scholar] [CrossRef]
- Kuttenberger, J.J.; Hardt, N. Long-term results following reconstruction of craniofacial defects with titanium micro-mesh systems. J. Craniomaxillofac. Surg. 2001, 29, 75–81. [Google Scholar] [CrossRef]
- Adamopoulos, O.; Papadopoulos, T. Nanostructured bioceramics for maxillofacial applications. J. Mater. Sci. Mater. Med. 2007, 18, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Niechajev, I. Facial reconstruction using porous high-density polyethylene (medpor): Long-term results. Aesthetic Plast. Surg. 2012, 36, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Martín, M.; Arias, J.; Carceller, F. Clinical applications of Norian SRS (calcium phosphate cement) in craniofacial reconstruction in children: Our experience at Hospital La Paz since 2001. J. Oral Maxillofac. Surg. 2005, 63, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Yoshimura, Y.; Nakanishi, Y.; Kanno, T.; Sano, H.; Kamei, Y. Anterior cranial base reconstruction using a hydroxyapatite-tricalciumphosphate composite (Ceratite®) as a bone substitute. J. Cranio-Maxillofac. Surg. 1995, 23, 64–67. [Google Scholar] [CrossRef]
- Kawecki, F.; Clafshenkel, W.P.; Fortin, M.; Auger, F.A.; Fradette, J. Biomimetic Tissue-engineered bone substitutes for maxillofacial and craniofacial repair: The potential of cell sheet technologies. Adv. Healthc. Mater. 2018, 7, 1700919. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Joint J. 2016, 98, 6–9. [Google Scholar] [CrossRef]
- Sándor, G.K.; Numminen, J.; Wolff, J.; Thesleff, T.; Miettinen, A.; Tuovinen, V.J.; Mannerström, B.; Patrikoski, M.; Seppänen, R.; Miettinen, S.; et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl. Med. 2014, 3, 530–540. [Google Scholar] [CrossRef]
- Thesleff, T.; Lehtimäki, K.; Niskakangas, T.; Huovinen, S.; Mannerström, B.; Miettinen, S.; Seppänen-Kaijansinkko, R.; Öhman, J. Cranioplasty with adipose-derived stem cells, β-tricalcium phosphate granules and supporting mesh: Six-year clinical follow-up results. Stem Cells Transl. Med. 2017, 6, 1576–1582. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J.; Little, D.G.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cells Mater. 2013, 26, 120–132. [Google Scholar] [CrossRef]
- Khojasteh, A.; Kheiri, L.; Behnia, H.; Tehranchi, A.; Nazeman, P.; Nadjmi, N.; Soleimani, M. Lateral ramus cortical bone plate in alveolar cleft osteoplasty with concomitant use of buccal fat pad derived cells and autogenous bone: Phase I clinical trial. Biomed Res. Int. 2017, 2017, 6560234. [Google Scholar] [CrossRef]
- Wolff, J.; Sándor, G.K.; Miettinen, A.; Tuovinen, V.J.; Mannerström, B.; Patrikoski, M.; Miettinen, S. GMP-level adipose stem cells combined with computer-aided manufacturing to reconstruct mandibular ameloblastoma resection defects: Experience with three cases. Ann. Maxillofac. Surg. 2013, 3, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, V.; Helder, M.N.; Bravenboer, N.; Ten Bruggenkate, C.M.; Jin, J.; Klein-Nulend, J.; Schulten, E.A. Bone tissue regeneration in the oral and maxillofacial region: A review on the application of stem cells and new strategies to improve vascularization. Stem Cells Int. 2019, 2019, 6279721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerem, R.M.; Sambanis, A. Tissue engineering: From biology to biological substitutes. Tissue Eng. 1995, 1, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [Green Version]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2015, 83, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B. Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [Green Version]
- Pirraco, R.P.; Iwata, T.; Yoshida, T.; Marques, A.P.; Yamato, M.; Reis, R.L.; Okano, T. Endothelial cells enhance the in vivo bone-forming ability of osteogenic cell sheets. Lab. Investig. 2014, 94, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Frohlich, M.; Grayson, W.; Wan, L.; Marolt, D.; Drobnic, M.; Vunjak-Novakovic, G. Tissue Engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Curr. Stem Cell Res. Ther. 2008, 3, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Laschke, M.W.; Menger, M.D. Prevascularization in tissue engineering: Current concepts and future directions. Biotechnol. Adv. 2016, 34, 112–121. [Google Scholar] [CrossRef]
- Gibot, L.; Galbraith, T.; Huot, J.; Auger, F.A. A preexisting microvascular network benefits in vivo revascularization of a microvascularized tissue-engineered skin substitute. Tissue Eng. Part A 2010, 16, 3199–3206. [Google Scholar] [CrossRef]
- Tremblay, P.L.; Hudon, V.; Berthod, F.; Germain, L.; Auger, F.A. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am. J. Transplant. 2005, 5, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, T.; Clafshenkel, W.P.; Kawecki, F.; Blanckaert, C.; Labbé, B.; Fortin, M.; Auger, F.A.; Fradette, J. A cell-based self-assembly approach for the production of human osseous tissues from adipose-derived stromal/stem cells. Adv. Healthc. Mater. 2017, 6, 1600889. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, F.; Clafshenkel, W.P.; Auger, F.A.; Bourget, J.M.; Fradette, J.; Devillard, R. Self-assembled human osseous cell sheets as living biopapers for the laser-assisted bioprinting of human endothelial cells. Biofabrication 2018, 10, 035006. [Google Scholar] [CrossRef] [PubMed]
- Vermette, M.; Trottier, V.; Ménard, V.; Saint-Pierre, L.; Roy, A.; Fradette, J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 2007, 28, 2850–2860. [Google Scholar] [CrossRef]
- Mayrand, D.; Laforce-Lavoie, A.; Larochelle, S.; Langlois, A.; Genest, H.; Roy, M.; Moulin, V.J. Angiogenic properties of myofibroblasts isolated from normal human skin wounds. Angiogenesis 2012, 15, 199–212. [Google Scholar] [CrossRef]
- Black, A.F.; Berthod, F.; L’heureux, N.; Germain, L.; Auger, F.A. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998, 12, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, C.J.; Fradette, J.; Galbraith, T.; Rémy, M.; Guignard, R.; Gauvin, R.; Germain, L.; Auger, F.A. Harvesting the potential of the human umbilical cord: Isolation and characterisation of four cell types for tissue engineering applications. Cells Tissues Org. 2012, 197, 37–54. [Google Scholar] [CrossRef]
- Chevalier, F.; Lavergne, M.; Negroni, E.; Ferratge, S.; Carpentier, G.; Gilbert-Sirieix, M.; Siñeriz, F.; Uzan, G.; Albanese, P. Glycosaminoglycan mimetic improves enrichment and cell functions of human endothelial progenitor cell colonies. Stem Cell Res. 2014, 12, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Germain, L.; Guignard, R.; Rouabhia, M.; Auger, F.A. Early basement membrane formation following the grafting of cultured epidermal sheets detached with thermolysin or dispase. Burns 1995, 21, 175–180. [Google Scholar] [CrossRef]
- Gibot, L.; Galbraith, T.; Bourland, J.; Rogic, A.; Skobe, M.; Auger, F.A. Tissue-engineered 3D human lymphatic microvascular network for in vitro studies of lymphangiogenesis. Nat. Protoc. 2017, 12, 1077–1088. [Google Scholar] [CrossRef]
- Mayrand, D.; Fradette, J. High definition confocal imaging modalities for the characterization of tissue-engineered substitutes. Methods Molec. Biol. 2018, 1773, 93–105. [Google Scholar]
- Chapoteau, E.; Czech, B.P.; Zazulak, W.; Kumar, A. New reagent for colorimetric assay of calcium in serum. Clin. Chem. 1993, 39, 1820–1824. [Google Scholar] [CrossRef]
- Chan, D.; Lamande, S.R.; Cole, W.G.; Bateman, J.F. Regulation of procollagen synthesis and processing during ascorbate-induced extracellular matrix accumulation in vitro. Biochem. J. 1990, 269, 175–181. [Google Scholar] [CrossRef]
- Auger, F.A.; Rémy-Zolghadri, M.; Grenier, G.; Germain, L. A Truly new approach for tissue engineering: The LOEX Self-assembly technique. In Stem Cell Transplantation and Tissue Engineering; Haverich, A., Graf, H., Eds.; Springer: Berlin, Germany, 2002; pp. 73–88. [Google Scholar]
- Emami, A.; Talaei-Khozani, T.; Vojdani, Z.; Zarei fard, N. Comparative assessment of the efficiency of various decellularization agents for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 19–32. [Google Scholar] [CrossRef]
- Rajab, T.K.; O’Malley, T.J.; Tchantchaleishvili, V. Decellularized scaffolds for tissue engineering: Current status and future perspective. Artif. Org. 2020, 44, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Nör, J.E.; Peters, M.C.; Christensen, J.B.; Sutorik, M.M.; Linn, S.; Khan, M.K.; Addison, C.L.; Mooney, D.J.; Polverini, P.J. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab. Investig. 2001, 81, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Maggiano, I.S.; Maggiano, C.M.; Clement, J.G.; Thomas, C.D.L.; Carter, Y.; Cooper, D.M.L. Three-dimensional reconstruction of Haversian systems in human cortical bone using synchrotron radiation-based micro-CT: Morphology and quantification of branching and transverse connections across age. J. Anat. 2016, 228, 719–732. [Google Scholar] [CrossRef] [Green Version]
- Grellier, M.; Bordenave, L.; Amédée, J. Cell-to-cell communication between osteogenic and endothelial lineages: Implications for tissue engineering. Trends Biotechnol. 2009, 27, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.C.; Mendes, L.F.; van Gastel, N.; Carmeliet, G.; Luyten, F.P. Fine-tuning pro-angiogenic effects of cobalt for simultaneous enhancement of vascular endothelial growth factor secretion and implant neovascularization. Acta Biomater. 2018, 72, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.R.; Oliveira, C.; Fernandes, E.M.; Reis, R.L.; Silva, T.H.; Martins, A.; Neves, N.M. Spatial immobilization of endogenous growth factors to control vascularization in bone tissue engineering. Biomater. Sci. 2020, 8, 2577–2589. [Google Scholar] [CrossRef]
- Cascone, I.; Audero, E.; Giraudo, E.; Napione, L.; Maniero, F.; Philips, M.R.; Collard, J.G.; Serini, G.; Bussolino, F. Tie-2-dependent activation of RhoA and Rac1 participates in endothelial cell motility triggered by angiopoietin-1. Blood 2003, 102, 2482–2490. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Li, Q.; Yang, D.; Fan, Z.; Ma, D.; Ren, L. Effect of different cell sheet ECM microenvironment on the formation of vascular network. Tissue Cell 2016, 48, 442–451. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1788–1798. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocalcin: Skeletal and extra-skeletal effects. J. Cell. Physiol. 2013, 228, 1149–1153. [Google Scholar] [CrossRef]
- Bouletreau, P.J.; Warren, S.M.; Spector, J.A.; Peled, Z.M.; Gerrets, R.P.; Greenwald, J.A.; Longaker, M.T. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: Implications for fracture healing. Plast. Reconstr. Surg. 2002, 109, 2384–2397. [Google Scholar] [CrossRef]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [Green Version]
- Meury, T.; Verrier, S.; Alini, M. Human endothelial cells inhibit BMSC differentiation into mature osteoblasts in vitro by interfering with osterix expression. J. Cell. Biochem. 2006, 98, 992–1006. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; De Crombrugghe, B. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Roux, B.M.; Vaicik, M.K.; Shrestha, B.; Montelongo, S.; Stojkova, K.; Yang, F.; Guda, T.; Cinar, A.; Brey, E.M. Induced Pluripotent stem cell-derived endothelial networks accelerate vascularization but not bone regeneration. Tissue Eng. Part A 2020. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sahar, D.E.; Walker, J.A.; Wang, H.T.; Stephenson, S.M.; Shah, A.R.; Krishnegowda, N.K.; Wenke, J.C. Effect of endothelial differentiated adipose-derived stem cells on vascularity and osteogenesis in poly (D,L-Lactide) scaffolds in vivo. J. Craniofac. Surg. 2012, 23, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Colavite, P.M.; Vieira, A.E.; Palanch Repeke, C.E.; de Araujo Linhari, R.P.; De Andrade, R.G.C.S.; Borrego, A.; De Franco, M.; Trombone, A.P.F.; Garlet, G.P. Alveolar bone healing in mice genetically selected in the maximum (AIRmax) or minimum (AIRmin) inflammatory reaction. Cytokine 2019, 114, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, E.; Leblanc, E.; Drevelle, O.; Giguère, R.; Beauvais, S.; Grenier, G.; Faucheux, N. The evaluation of ectopic bone formation induced by delivery systems for bone morphogenetic protein-9 or its derived peptide. Tissue Eng. Part A 2012, 18, 342–352. [Google Scholar] [CrossRef]
- Luu, H.H.; Song, W.X.; Luo, X.; Manning, D.; Luo, J.; Deng, Z.L.; Sharff, K.A.; Montag, A.G.; Haydon, R.C.; He, T.C. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J. Orthop. Res. 2007, 25, 665–677. [Google Scholar] [CrossRef]
- Song, I.; Kim, B.S.; Kim, C.S.; Im, G. Il Effects of BMP-2 and vitamin D3 on the osteogenic differentiation of adipose stem cells. Biochem. Biophys. Res. Commun. 2011, 408, 126–131. [Google Scholar] [CrossRef]
- Yanai, R.; Tetsuo, F.; Ito, S.; Itsumi, M.; Yoshizumi, J.; Maki, T.; Mori, Y.; Kubota, Y.; Kajioka, S. Extracellular calcium stimulates osteogenic differentiation of human adipose-derived stem cells by enhancing bone morphogenetic protein-2 expression. Cell Calcium 2019, 83, 102058. [Google Scholar] [CrossRef]
- Vanhatupa, S.; Ojansivu, M.; Autio, R.; Juntunen, M.; Miettinen, S. Bone morphogenetic protein-2 induces donor-dependent osteogenic and adipogenic differentiation in human adipose stem cells. Stem Cells Transl. Med. 2015, 4, 1391–1402. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Wang, Y.; Yan, W.; Li, H.; Shi, Z.; Pan, Z. Combined mesenchymal stem cell sheets and rhBMP-2-releasing calcium sulfate-rhBMP-2 scaffolds for segmental bone tissue engineering. Cell Transplant 2012, 21, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, O.; Sheyn, D.; Tawackoli, W.; Kallai, I.; Oh, A.; Su, S.; Da, X.; Zarrini, P.; Cook-Wiens, G.; Gazit, D.; et al. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther. 2013, 20, 370–377. [Google Scholar] [CrossRef] [PubMed]








| D3 to D10 | D10 to D21 | D21 to D35 | |
|---|---|---|---|
| Osteogenic medium for bone-like substitutes + HUVEC and − HUVEC (final concentration) | Dulbecco’s modified Eagle’s medium (DMEM) | DMEM | DMEM |
| Fetal calf serum (FCS) (10%) | FCS (10%) | FCS (5%) | |
| Penicillin (100 U mL−1) | Penicillin (100 U mL−1) | Penicillin (100 U mL−1) | |
| Gentamicin (25 μg mL−1) | Gentamicin (25 μg mL−1) | Gentamicin (25 μg mL−1) | |
| Calcium chloride (1.8 × 10−3 M) | Calcium chloride (1.8 × 10−3 M) | Calcium chloride (1.8 × 10−3 M) | |
| Sodium L-ascorbic acid (50 μg mL−1) | Sodium L-ascorbic acid (50 μg mL−1) | Sodium L-ascorbic acid (50 μg mL−1) | |
| Dexamethasone (10 × 10−9 M) | Dexamethasone (10 × 10−9 M) | Dexamethasone (10 × 10−9 M) | |
| 1α,25-dihydroxyvitamin D3 (10 × 10−9 M) | 1α,25-dihydroxyvitamin D3 (10 × 10−9 M) | 1α,25-dihydroxyvitamin D3 (10 × 10−9 M) | |
| Ascorbate-2-phosphate (50 × 10−6 M) | Ascorbate-2-phosphate (50 × 10−6 M) | Ascorbate-2-phosphate (50 × 10−6 M) | |
| ß-glycerophosphate (3.5 × 10−3 M) | ß-glycerophosphate (3.5 × 10−3 M) | ||
| Endothelial cell growth basal medium-2 (EBM-2TM; CC-3156) | |||
| EGMTM-2 MV SingleQuotsTM Supplement Pack (CC-4147): Fetal bovine serum (FBS) (2.5%) Hydrocortisone Human basic FGF Vascular endothelial growth factor (VEGF) R3-IGF-1 Human EGF | |||
| Basal medium for stromal substitutes + HUVEC and − HUVEC (final concentration) | DMEM | DMEM | DMEM |
| FCS (10%) | FCS (10%) | FCS (5%) | |
| Penicillin (100 U mL−1) | Penicillin (100 U mL−1) | Penicillin (100 U mL−1) | |
| Gentamicin (25 μg mL−1) | Gentamicin (25 μg mL−1) | Gentamicin (25 μg mL−1) | |
| Calcium chloride (1.8 × 10−3 M) | Calcium chloride (1.8 × 10−3 M) | Calcium chloride (1.8 × 10−3 M) | |
| Sodium L-ascorbic acid (50 μg mL−1) | Sodium L-ascorbic acid (50 μg mL−1) | Sodium L-ascorbic acid (50 μg mL−1) | |
| EBM-2TM | |||
| EGMTM-2 MV SingleQuotsTM Supplement Pack |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawecki, F.; Galbraith, T.; Clafshenkel, W.P.; Fortin, M.; Auger, F.A.; Fradette, J. In Vitro Prevascularization of Self-Assembled Human Bone-Like Tissues and Preclinical Assessment Using a Rat Calvarial Bone Defect Model. Materials 2021, 14, 2023. https://doi.org/10.3390/ma14082023
Kawecki F, Galbraith T, Clafshenkel WP, Fortin M, Auger FA, Fradette J. In Vitro Prevascularization of Self-Assembled Human Bone-Like Tissues and Preclinical Assessment Using a Rat Calvarial Bone Defect Model. Materials. 2021; 14(8):2023. https://doi.org/10.3390/ma14082023
Chicago/Turabian StyleKawecki, Fabien, Todd Galbraith, William P. Clafshenkel, Michel Fortin, François A. Auger, and Julie Fradette. 2021. "In Vitro Prevascularization of Self-Assembled Human Bone-Like Tissues and Preclinical Assessment Using a Rat Calvarial Bone Defect Model" Materials 14, no. 8: 2023. https://doi.org/10.3390/ma14082023
APA StyleKawecki, F., Galbraith, T., Clafshenkel, W. P., Fortin, M., Auger, F. A., & Fradette, J. (2021). In Vitro Prevascularization of Self-Assembled Human Bone-Like Tissues and Preclinical Assessment Using a Rat Calvarial Bone Defect Model. Materials, 14(8), 2023. https://doi.org/10.3390/ma14082023