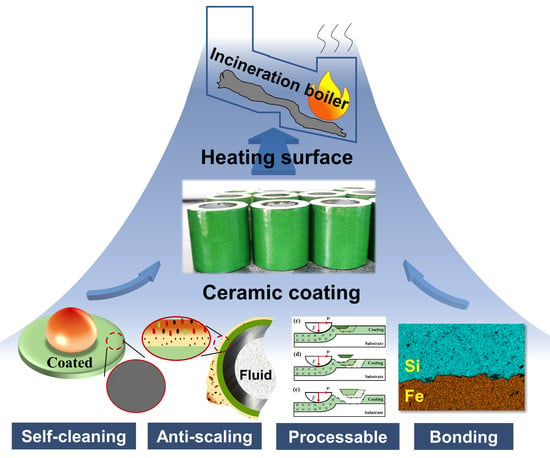
Properties of Ceramic Coating on Heating Surface of Waste Incineration Boiler Prepared by Slurry Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Slurry Preparation
2.2. Coating Preparation
2.3. Mechanical Properties Evaluation
2.3.1. Evaluation of Thermal Shock Resistance
2.3.2. Bonding Performance Test
2.4. High Temperature Wettability Test
3. Results and Discussion
3.1. Coating Morphology
3.2. Mechanical Properties
3.3. High-Temperature Wetting Performance
4. Conclusions
- (1)
- Ceramic coatings can be prepared by the slurry method and in an environment simulating a start-up boiler. They can be sintered at a low temperature of 750 °C. The surface and cross-section are dense, the porosity is less than 1%, and the bonding strength is 25.14 ± 2.21 MPa.
- (2)
- The thermal shock test showed that the coating peeled off about 5% of the coating after 60 cycles at 700 °C. The results of the microscratch test showed that the coating is not be completely separated after being subjected to pressure loading. The failure mode is wedging spalling due to cohesive failure, and coated products are machinable. The mechanical property test showed that the coating meets the primary requirements of practical applications.
- (3)
- The high-temperature wettability experiment results showed that the ceramic coating has a smaller liquid-bridge force, smaller adhesion area, shorter fouling cycle for molten corrosive fouling, and has good antiscaling properties, and the potential for self-cleaning.
- (4)
- The hydrophobic theory applied to normal temperature still plays a guiding role in studying the wettability of complex liquids in high-temperature environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, B.; Gan, M.; Ji, Z.; Fan, X.; Zhang, D.; Chen, X.; Sun, Z.; Huang, X.; Fan, Y. Recent progress on the thermal treatment and resource utilization technologies of municipal waste incineration fly ash: A review. Process Saf. Environ. Prot. 2022, 159, 547–565. [Google Scholar] [CrossRef]
- Torrell, M.; Dosta, S.; Miguel, J.R.; Guilemany, J.M. Guilemany, Optimisation of HVOF thermal spray coatings for their implementation as MSWI superheater protectors. Corros. Eng. Sci. Technol. 2010, 45, 84–93. [Google Scholar] [CrossRef]
- Makarichi, L.; Jutidamrongphan, W.; Techato, K. The evolution of waste-to-energy incineration: A review. Renew. Sustain. Energy Rev. 2018, 91, 812–821. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, X. Detoxification, solidification and recycling of municipal solid waste incineration fly ash: A review. Chem. Eng. J. 2021, 420, 130349. [Google Scholar] [CrossRef]
- Wang, P.; Hu, Y.; Cheng, H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ. Pollut. 2019, 252, 461–475. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, Z.; Luo, Z.; Yu, L.; Yin, K. Phase changes during various treatment processes for incineration bottom ash from municipal solid wastes: A review in the application-environment nexus. Environ. Pollut. 2021, 287, 117618. [Google Scholar] [CrossRef]
- Padmini, B.V.; Bhosale, D.G.; Niranjan, H.B. A study of T11 boiler steel protection by cold sprayed Inconel 738 coating against high temperature erosion. Surf. Interfaces 2021, 23, 101002. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Wang, Y.; Tang, J. A comparative study on the high temperature corrosion of TP347H stainless steel, C22 alloy and laser-cladding C22 coating in molten chloride salts. Corros. Sci. 2014, 83, 396–408. [Google Scholar] [CrossRef]
- Bala, N.; Singh, H.; Prakash, S. Performance of cold sprayed Ni based coatings in actual boiler environment. Surf. Coat. Technol. 2017, 318, 50–61. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, Y.; Chi, Z.; Zhang, G. High-temperature sulfur corrosion behavior of h-BN-based ceramic coating prepared by slurry method. Mater. Chem. Phys. 2018, 206, 186–192. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Li, F.; Chi, Z. Lyophobicity and slagging resistance mechanism of h-BN based coating for coal-fired boilers. Fuel Process. Technol. 2019, 188, 43–50. [Google Scholar] [CrossRef]
- Bläsing, M.; Müller, M. Investigation of the effect of alkali metal sorbents on the release and capture of trace elements during combustion of straw. Combust. Flame 2013, 160, 3015–3020. [Google Scholar] [CrossRef]
- Wu, H.; Bashir, M.S.; Jensen, P.A.; Sander, B.; Glarborg, P. Impact of coal fly ash addition on ash transformation and deposition in a full-scale wood suspension-firing boiler. Fuel 2013, 113, 632–643. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, R.; Liu, J.; Bhattacharya, S. Performance of Fe2O3/CaSO4 composite oxygen carrier on inhibition of sulfur release in calcium-based chemical looping combustion. Int. J. Greenh. Gas Control 2013, 17, 1–12. [Google Scholar] [CrossRef]
- Duriagina, Z.; Kovbasyuk, T.; Zagula-Yavorska, M.; Bespalov, S.; Drajewicz, M.; Dychton, K.; Kindrachuk, M. Comparative Estimation of the Structure and Electrical Properties of Functional Layers Based on PbO–ZnO–B2O3 Glass–Ceramic Sealant. Powder Metall. Met. Ceram. 2017, 55, 580–584. [Google Scholar] [CrossRef]
- Qian, H.; Xu, Z.; Chen, S.; Liu, Y.; Yan, D. Silicon carbide/enamel composite coatings for steel corrosion protection: Microstructure, thermal expansion behavior, and anti-corrosion performance. Surf. Coat. Technol. 2022, 434, 128172. [Google Scholar] [CrossRef]
- Baitalik, S.; Kayal, N. Thermal shock and chemical corrosion resistance of oxide bonded porous SiC ceramics prepared by infiltration technique. J. Alloys Compd. 2019, 781, 289–301. [Google Scholar] [CrossRef]
- Patra, L.; Pandey, R. Mechanical properties of 2D materials: A review on molecular dynamics based nanoindentation simulations. Mater. Today Commun. 2022, 31, 103623. [Google Scholar] [CrossRef]
- Goswami, M.; Sarkar, A.; Mirza, T.; Shrikhande, V.K.; Sangeeta; Gurumurthy, K.R.; Kothiyal, G.P. Study of some thermal and mechanical properties of magnesium aluminium silicate glass ceramic. Ceram. Int. 2002, 28, 585–592. [Google Scholar] [CrossRef]
- Molla, A.R.; Basu, B. Microstructure, mechanical, and in vitro properties of mica glass-ceramics with varying fluorine content. J. Mater. Sci. Mater. Med. 2009, 20, 869–882. [Google Scholar] [CrossRef]
- Chen, J.; Ninomiya, Y.; Naganuma, H.; Sasaki, Y.; Noguchi, M.; Cho, H.; Ueki, Y.; Yoshiie, R.; Naruse, I. Development of thermal spraying materials through several corrosion tests for heat exchanger tube of incinerators. Fuel Process. Technol. 2016, 141, 216–224. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Zhang, D. Recent advances in chemical durability and mechanical stability of superhydrophobic materials: Multi-strategy design and strengthening. J. Mater. Sci. Technol. 2022, 129, 40–69. [Google Scholar] [CrossRef]
- Nyankson, E.; Agbe, H.; Takyi, G.K.S.; Bensah, Y.D.; Sarkar, D.K. Recent advances in nanostructured superhydrophobic surfaces: Fabrication and long-term durability challenges. Curr. Opin. Chem. Eng. 2022, 36, 100790. [Google Scholar] [CrossRef]
- Li, W.; Zhan, Y.; Yu, S. Applications of superhydrophobic coatings in anti-icing: Theory, mechanisms, impact factors, challenges and perspectives. Prog. Org. Coat. 2021, 152, 106117. [Google Scholar] [CrossRef]












| Raw Material | Mica | Copper | Chromium Oxide | Graphite | Sodium Silicate | Water | Dispersant | Defoamer |
|---|---|---|---|---|---|---|---|---|
| wt.% | 10 | 5 | 5 | 3 | 65 | 10 | 1 | 1 |
| Corrosive Mixture | NaCl | KCl | Na2SO4 | K2SO4 | PbO | ZnO |
|---|---|---|---|---|---|---|
| wt.% | 20 | 20 | 20 | 20 | 10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Wu, L.; Liang, X. Properties of Ceramic Coating on Heating Surface of Waste Incineration Boiler Prepared by Slurry Method. Materials 2022, 15, 4574. https://doi.org/10.3390/ma15134574
Wei Z, Wu L, Liang X. Properties of Ceramic Coating on Heating Surface of Waste Incineration Boiler Prepared by Slurry Method. Materials. 2022; 15(13):4574. https://doi.org/10.3390/ma15134574
Chicago/Turabian StyleWei, Zengzhi, Lijun Wu, and Xingyuan Liang. 2022. "Properties of Ceramic Coating on Heating Surface of Waste Incineration Boiler Prepared by Slurry Method" Materials 15, no. 13: 4574. https://doi.org/10.3390/ma15134574
APA StyleWei, Z., Wu, L., & Liang, X. (2022). Properties of Ceramic Coating on Heating Surface of Waste Incineration Boiler Prepared by Slurry Method. Materials, 15(13), 4574. https://doi.org/10.3390/ma15134574