Environmental STEM Study of the Oxidation Mechanism for Iron and Iron Carbide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Iron Carbide Nanoparticles
2.3. Synthesis of Iron Nanoparticles
2.4. Environmental Scanning Transmission Electron Microscopy
2.5. Scanning Transmission Electron Microscopy
3. Results
3.1. Characterization of Fe and Fe2C Nanoparticles
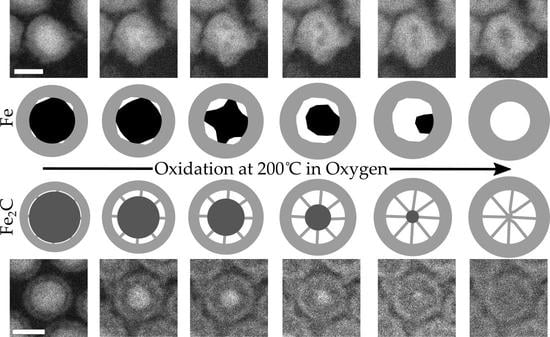
3.2. ESTEM Imaging of Fe Nanoparticles
3.3. ESTEM Imaging of Fe2C Nanoparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huber, D.L. Synthesis, Properties, and Applications of Iron Nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef]
- Ferguson, P.M.; Feindel, K.W.; Slocombe, A.; MacKay, M.; Wignall, T.; Delahunt, B.; Tilley, R.D.; Hermans, I.F. Strongly Magnetic Iron Nanoparticles Improve the Diagnosis of Small Tumours in the Reticuloendothelial System by Magnetic Resonance Imaging. PLoS ONE 2013, 8, e56572. [Google Scholar] [CrossRef]
- Herman, D.A.J.; Ferguson, P.; Cheong, S.; Hermans, I.F.; Ruck, B.J.; Allan, K.M.; Prabakar, S.; Spencer, J.L.; Lendrum, C.D.; Tilley, R.D. Hot-Injection Synthesis of Iron/Iron Oxide Core/Shell Nanoparticles for T2 Contrast Enhancement in Magnetic Resonance Imaging. Chem. Commun. 2011, 47, 9221–9223. [Google Scholar] [CrossRef] [PubMed]
- Gloag, L.; Mehdipour, M.; Ulanova, M.; Mariandry, K.; Nichol, M.A.; Hernández-Castillo, D.J.; Gaudet, J.; Qiao, R.; Zhang, J.; Nelson, M.; et al. Zero Valent Iron Core–Iron Oxide Shell Nanoparticles as Small Magnetic Particle Imaging Tracers. Chem. Commun. 2020, 56, 3504–3507. [Google Scholar] [CrossRef]
- Famiani, S.; LaGrow, A.P.; Besenhard, M.O.; Maenosono, S.; Thanh, N.T.K. Synthesis of Fine-Tuning Highly Magnetic Fe@Fe x O y Nanoparticles through Continuous Injection and a Study of Magnetic Hyperthermia. Chem. Mater. 2018, 30, 8897–8904. [Google Scholar] [CrossRef]
- Yadong, Y.; Rioux, R.M.; Erdonmez, C.K.; Steven, H.; Somorjai, G.A.; Paul, A.A. Formation of Hollow Nanocrystals Through the Nanoscale Kirkendall Effect. Science 2004, 304, 711–714. [Google Scholar]
- McGrath, A.J.; Dolan, C.; Cheong, S.; Herman, D.A.J.; Naysmith, B.; Zong, F.; Galvosas, P.; Farrand, K.J.; Hermans, I.F.; Brimble, M.; et al. Stability of Polyelectrolyte-Coated Iron Nanoparticles for T2-Weighted Magnetic Resonance Imaging. J. Magn. Magn. Mater. 2017, 439, 251–258. [Google Scholar] [CrossRef]
- Loizou, K.; Mourdikoudis, S.; Sergides, A.; Besenhard, M.O.; Sarafidis, C.; Higashimine, K.; Kalogirou, O.; Maenosono, S.; Thanh, N.T.K.; Gavriilidis, A. Rapid Millifluidic Synthesis of Stable High Magnetic Moment FexCy Nanoparticles for Hyperthermia. ACS Appl. Mater. Interfaces 2020, 12, 28520–28531. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Chun, D.H.; Yang, J.-I.; Jung, H.; Lee, H.-T.; Hong, S.; Jang, S.; Lim, J.T.; Kim, C.S.; Park, J.C. A New Synthesis of Carbon Encapsulated Fe5C2 Nanoparticles for High-Temperature Fischer–Tropsch Synthesis. Nanoscale 2015, 7, 16616–16620. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, H.; Hou, Y.; Ma, D. Fe5C2 Nanoparticles: A Facile Bromide-Induced Synthesis and as an Active Phase for Fischer-Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef]
- Xu, K.; Sun, B.; Lin, J.; Wen, W.; Pei, Y.; Yan, S.; Qiao, M.; Zhang, X.; Zong, B. ε-Iron Carbide as a Low-Temperature Fischer–Tropsch Synthesis Catalyst. Nat. Commun. 2014, 5, 5783. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Cao, Z.; Zhao, S.; Gao, R.; Meng, Y.; Zhu, J.X.; Rogers, C.; Huo, C.F.; Yang, Y.; Li, Y.W.; et al. Iron Carbides in Fischer-Tropsch Synthesis: Theoretical and Experimental Understanding in Epsilon-Iron Carbide Phase Assignment. J. Phys. Chem. C 2017, 121, 21390–21396. [Google Scholar] [CrossRef]
- Tang, W.; Zhen, Z.; Yang, C.; Wang, L.; Cowger, T.; Chen, H.; Todd, T.; Hekmatyar, K.; Zhao, Q.; Hou, Y.; et al. Fe5C2 Nanoparticles with High Mri Contrast Enhancement for Tumor Imaging. Small 2014, 10, 1245–1249. [Google Scholar] [CrossRef]
- Huang, G.; Hu, J.; Zhang, H.; Zhou, Z.; Chi, X.; Gao, J. Highly Magnetic Iron Carbide Nanoparticles as Effective T2 Contrast Agents. Nanoscale 2014, 6, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, C.; Li, J.; Ding, Y.; Zhang, L.; Yousaf, M.Z.; Lin, J.; Pang, R.; Wei, L.; Xu, L.; et al. Multifunctional Fe5C2 Nanoparticles: A Targeted Theranostic Platform for Magnetic Resonance Imaging and Photoacoustic Tomography-Guided Photothermal Therapy. Adv. Mater. 2014, 26, 4114–4120. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ju, Y.; Zhao, L.; Chu, X.; Yang, W.; Tian, Y.; Sheng, F.; Lin, J.; Liu, F.; Dong, Y.; et al. Multistimuli-Regulated Photochemothermal Cancer Therapy Remotely Controlled via Fe5C2 Nanoparticles. ACS Nano 2016, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, F.; Gao, W.; Ju, Y.; Chu, X.; Che, S.; Sheng, F.; Hou, Y. Iron Carbide Nanoparticles: An Innovative Nanoplatform for Biomedical Applications. Nanoscale Horiz. 2017, 2, 81–88. [Google Scholar] [CrossRef]
- Bordet, A.; Lacroix, L.-M.; Fazzini, P.-F.; Carrey, J.; Soulantica, K.; Chaudret, B. Magnetically Induced Continuous CO2 Hydrogenation Using Composite Iron Carbide Nanoparticles of Exceptionally High Heating Power. Angew. Chem. 2016, 55, 15894–15898. [Google Scholar] [CrossRef]
- Cabot, A.; Puntes, V.F.; Shevchenko, E.; Yin, Y.; Balcells, L.; Marcus, M.A.; Hughes, S.M.; Alivisatos, A.P. Vacancy Coalescence during Oxidation of Iron Nanoparticles. J. Am. Chem. Soc. 2007, 129, 10358–10360. [Google Scholar] [CrossRef]
- Yugang, S.; Xiaobing, Z.; Sankaranarayanan, S.K.; Sheng, P.; Badri, N.; Ganesh, K. Quantitative 3D Evolution of Colloidal Nanoparticle Oxidation in Solution. Science 2017, 356, 303–307. [Google Scholar]
- Boyes, E.D.; Gai, P.L. Environmental High Resolution Electron Microscopy and Applications to Chemical Science. Ultramicroscopy 1997, 67, 219–232. [Google Scholar] [CrossRef]
- Chenna, S.; Crozier, P.A. In Situ Environmental Transmission Electron Microscopy to Determine Transformation Pathways in Supported Ni Nanoparticles. Micron 2012, 43, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.W.; Wagner, J.B.; Hansen, P.L.; Dahl, S.; Topsøe, H.; Jacobsen, C.J. Atomic-Resolution in Situ Transmission Electron Microscopy of a Promoter of a Heterogeneous Catalyst. Science 2001, 294, 1508–1510. [Google Scholar] [CrossRef]
- Janbroers, S.; Crozier, P.A.; Zandbergen, H.W.; Kooyman, P.J. A Model Study on the Carburization Process of Iron-Based Fischer–Tropsch Catalysts Using in Situ TEM–EELS. Appl. Catal. B Environ. 2011, 102, 521–527. [Google Scholar] [CrossRef]
- Gai, P.L.; Boyes, E.D. Advances in Atomic Resolution in Situ Environmental Transmission Electron Microscopy and 1Å Aberration Corrected in Situ Electron Microscopy. Microsc. Res. Tech. 2009, 72, 153–164. [Google Scholar] [CrossRef]
- Boyes, E.D.; LaGrow, A.P.; Ward, M.R.; Mitchell, R.W.; Gai, P.L. Single Atom Dynamics in Chemical Reactions. Acc. Chem. Res. 2020, 53, 390–399. [Google Scholar] [CrossRef] [PubMed]
- LaGrow, A.P.; Ward, M.R.; Lloyd, D.C.; Gai, P.L.; Boyes, E.D. Visualizing the Cu/Cu2O Interface Transition in Nanoparticles with Environmental Scanning Transmission Electron Microscopy. J. Am. Chem. Soc. 2017, 139, 179–185. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, G.; Filsinger, G.T.; Wu, L.; Zhu, H.; Lee, J.; Wu, Z.; Sun, S. Halide Ion-Mediated Growth of Single Crystalline Fe Nanoparticles. Nanoscale 2014, 6, 4852–4856. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Alyami, N.M.; Lloyd, D.C.; Bakr, O.M.; Boyes, E.D.; Gai, P.L. In Situ Oxidation and Reduction of Triangular Nickel Nanoplates via Environmental Transmission Electron Microscopy. J. Microsc. 2018, 269, 161–167. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, H.; Datye, A.K. Electron Energy Loss Spectroscopy (EELS) of Iron Fischer–Tropsch Catalysts. Microsc. Microanal. 2006, 12, 124–134. [Google Scholar] [CrossRef]
- Mann, D.K.; Xu, J.; Mordvinova, N.E.; Yannello, V.; Ziouani, Y.; Gonzalez-Ballesteros, N.; Sousa, J.P.S.; Lebedev, O.I.; Kolen’ko, Y.V.; Shatruk, M. Electrocatalytic water oxidation over AlFe2B2. Chem. Sci. 2019, 10, 2796. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Lloyd, D.C.; Schebarchov, D.; Gai, P.L.; Boyes, E.D. In Situ Visualization of Site-Dependent Reaction Kinetics in Shape-Controlled Nanoparticles: Corners vs Edges. J. Phys. Chem. C 2019, 123, 14746–14753. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Lloyd, D.C.; Gai, P.L.; Boyes, E.D. In Situ Scanning Transmission Electron Microscopy of Ni Nanoparticle Redispersion via the Reduction of Hollow NiO. Chem. Mater. 2018, 30, 197–203. [Google Scholar] [CrossRef]
- Wang, C.-M.; Genc, A.; Cheng, H.; Pullan, L.; Baer, D.R.; Bruemmer, S.M. In-Situ TEM Visualization of Vacancy Injection and Chemical Partition during Oxidation of Ni-Cr Nanoparticles. Sci. Rep. 2014, 4, 3683. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Yang, Y.; Meng, Q.; Deng, Z.; Gong, M.; Wang, J.; Wang, D.; Zhu, Y.; Sun, L.; Xu, F.; et al. Bimetallic Nanoparticle Oxidation in Three Dimensions by Chemically Sensitive Electron Tomography and in Situ Transmission Electron Microscopy. ACS Nano 2018, 12, 7866–7874. [Google Scholar] [CrossRef]
- Wang, C.M.; Baer, D.R.; Thomas, L.E.; Amonette, J.E.; Antony, J.; Qiang, Y.; Duscher, G. Void Formation during Early Stages of Passivation: Initial Oxidation of Iron Nanoparticles at Room Temperature. J. Appl. Phys. 2005, 98, 94308. [Google Scholar] [CrossRef]
- Wang, W.; Dahl, M.; Yin, Y. Hollow Nanocrystals through the Nanoscale Kirkendall Effect. Chem. Mater. 2013, 25, 1179–1189. [Google Scholar] [CrossRef]
- Freel, J.; Wheeler, B.R.; Galwey, A.K. Kinetic Study of the Oxidation of the Carbides of Iron. Trans. Faraday Soc. 1970, 66, 1015–1024. [Google Scholar] [CrossRef]
- Meng, S.; Wu, J.; Zhao, L.; Zheng, H.; Jia, S.; Hu, S.; Meng, W.; Pu, S.; Zhao, D.; Wang, J. Atomistic Insight into the Redox Reactions in Fe/Oxide Core-Shell Nanoparticles. Chem. Mater. 2018, 30, 7306–7312. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LaGrow, A.P.; Famiani, S.; Sergides, A.; Lari, L.; Lloyd, D.C.; Takahashi, M.; Maenosono, S.; Boyes, E.D.; Gai, P.L.; Thanh, N.T.K. Environmental STEM Study of the Oxidation Mechanism for Iron and Iron Carbide Nanoparticles. Materials 2022, 15, 1557. https://doi.org/10.3390/ma15041557
LaGrow AP, Famiani S, Sergides A, Lari L, Lloyd DC, Takahashi M, Maenosono S, Boyes ED, Gai PL, Thanh NTK. Environmental STEM Study of the Oxidation Mechanism for Iron and Iron Carbide Nanoparticles. Materials. 2022; 15(4):1557. https://doi.org/10.3390/ma15041557
Chicago/Turabian StyleLaGrow, Alec P., Simone Famiani, Andreas Sergides, Leonardo Lari, David C. Lloyd, Mari Takahashi, Shinya Maenosono, Edward D. Boyes, Pratibha L. Gai, and Nguyen Thi Kim Thanh. 2022. "Environmental STEM Study of the Oxidation Mechanism for Iron and Iron Carbide Nanoparticles" Materials 15, no. 4: 1557. https://doi.org/10.3390/ma15041557
APA StyleLaGrow, A. P., Famiani, S., Sergides, A., Lari, L., Lloyd, D. C., Takahashi, M., Maenosono, S., Boyes, E. D., Gai, P. L., & Thanh, N. T. K. (2022). Environmental STEM Study of the Oxidation Mechanism for Iron and Iron Carbide Nanoparticles. Materials, 15(4), 1557. https://doi.org/10.3390/ma15041557