Orodispersible Films with Rupatadine Fumarate Enclosed in Ethylcellulose Microparticles as Drug Delivery Platform with Taste-Masking Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
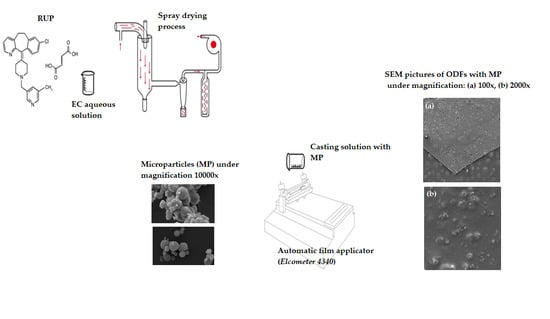
2.2. Preparation of MPs and ODFs
2.3. Viscosity Measurements
2.4. Evaluation of Morphology of ODFs
2.5. Quality Assessment of ODFs
2.5.1. Uniformity of Weight and Thickness
2.5.2. Moisture Content
2.5.3. Drug Content
2.5.4. Mechanical Properties
2.6. Disintegration Time Assessment—In Vivo in Healthy Volunteers, Petri Dish, Drop Method
2.7. Differential Scanning Calorimetry (DSC)
2.8. Evaluation of Taste-Masking Effectiveness
2.8.1. In Vivo in Healthy Volunteers
2.8.2. RUP Dissolution
2.8.3. Electronic Tongue
Membrane Materials
Fabrication of the Electrodes and Membrane Preparation
Electronic Tongue Measurements and Performance of the Electrodes
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, T.R.; Jamshaid, M.; Bashir, I.; Zulfiqar, S. Oral films: A comprehensive review. Int. Cur. Pharm. J. 2016, 5, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Scarpaa, M.; Paudelb, A.; Kloproggec, F.; Hsiaob, W.K.; Brescianib, M.; Gaisforda, S.; Orlua, M. Key acceptability attributes of orodispersible films. Eur. J. Pharm. Biopharm. 2018, 125, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Oral films: Current status and future perspectives I—galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cilurzo, F.; Musazzi, U.M.; Franze, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: Biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpa, M.; Stegemann, S.; Hsiao, W.K.; Pichler, H.; Gaisford, S.; Bresciani, M.; Paudel, A.; Orlu, M. Orodispersible films: Towards drug delivery in special populations. Int. J. Pharm. 2017, 523, 327–335. [Google Scholar] [CrossRef] [PubMed]
- The European Pharmacopoeia, 10th ed.; Council of Europe: Strasburg, France, 2019.
- FDA; CDER. Guidance for Industry—Orally Disintegrating Tablets. 2008. Available online: https://www.fda.gov/downloads/Drugs/Guidances/ucm070578.pdf (accessed on 10 January 2022).
- The United States Pharmacopeia and National Formulary (USP41-NF 36); Pharmacopeia Convention: Rockville, MD, USA, 2018; Volume 2.
- Olechno, K.; Basa, A.; Winnicka, K. “Success depends on your backbone” – about the use of polymers as essential materials forming orodispersible films. Materials 2021, 14, 4872. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Mane, M.; Ghadge, D. Exploration of different polymers for use in the formulation of oral fast dissolving strips. J. Cur. Pharm. Res. 2010, 2, 33–35. [Google Scholar]
- Liew, K.B.; Tan, Y.T.F.; Peh, K.-K. Effect of polymer, plasticizer and filler on orally disintegrating films. Drug Dev. Ind. Pharm. 2014, 40, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Faisal, W.; Farag, F.; Abdellatif, A.A.H.; Abbas, A. Taste masking approaches for medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, X.D.; Selomuyla, C. On the spray drying of uniform functional microparticles. Particuology 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Wasilewska, K.; Szekalska, M.; Ciosek-Skibinska, P.; Lenik, J.; Basa, A.; Jacyna, J.; Markuszewski, M.; Winnicka, K. Ethylcellulose in organic solution or aqueous dispersion form in designing taste-masked microparticles by the spray drying technique with a model bitter drug: Rupatadine fumarate. Polymers 2019, 11, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA; Washington, DC, USA, 2009. [Google Scholar]
- FDA Inactive Ingredients Database. Available online: https://search.fda.gov/search?utf8=%E2%9C%93&affiliate=fda1&query=ethylcellulose&commit=Search (accessed on 10 January 2022).
- Safety & Toxicity of Excipients for Paediatrics, STEP Database. Available online: http://www.eupfi.org/step-database-info/ (accessed on 10 January 2022).
- Shamizadeh, S.; Brockow, K.; Ring, J. Rupatadine: Efficacy and safety of a non-sedating antihistamine with PAF-antagonist effects. Allergo, J. Int. 2014, 23, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullol, J.; Gonzalez-Nunez, V.; Bachert, C. Rupatadine: Global safety evaluation in allergic rhinitis and urticaria. Expert Opin. Drug Saf. 2016, 15, 1439–1448. [Google Scholar]
- Rupatadine fumarate dosage. Available online: https://www.medicines.org.uk/emc/product/9888/smpc#gref (accessed on 10 January 2022).
- Preis, M.; Pein, M.; Breitkreutz, J. Development of a taste-masked orodispersible film containing dimenhydrinate. Pharmaceutics 2012, 4, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Choudekar, R.L.; Mahajan, M.P.; Sawant, S.D. Validated RP-HPLC method for the estimation of rupatadine fumarate in bulk and tablet dosage form. Pharma Chem. 2012, 4, 1047–1053. [Google Scholar]
- Redasani, V.K.; Kothawade, A.R.; Surana, S.J. Stability indicating RP-HPLC method for simultaneous estimation of rupatadine fumarate and montelukast sodium in bulk and tablet dosage form. J. Anal. Chem. 2014, 69, 384–389. [Google Scholar] [CrossRef]
- Rele, R.V.; Mali, R.N. New validated RP-HPLC method for quantification of rupatadine fumarate impurities in solid dosage form supported by forced degradation studies. Der Pharm. Lett. 2016, 8, 66–72. [Google Scholar]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Szymańska, E.; Wróblewska, M.; Kurowska, I.; Maciejczyk, M.; Basa, A.; Wolska, E.; Wilczewska, A.Z.; Winnicka, K. Multilayer films based on chitosan/pectin polyelectrolytecomplexes as novel platforms for buccal administration of clotrimazole. Pharmaceutics 2021, 13, 1558. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al–Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Chaiwarit, T.; Jantanasakulwong, K.; Brachais, C.H. Effect of plasticizer type on tensile property and in vitro indomethacin release of thin films based on low-methoxyl pectin. Polymers 2017, 9, 289. [Google Scholar]
- Łabańska, M.; Ciosek-Skibińska, P.; Wróblewski, W. Critical evaluation of laboratory potentiometric electronic tongues for pharmaceutical analysis—an overview. Sensors 2019, 19, 5376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose— a traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef] [PubMed]
- Woertz, C.; Kleinebudde, P. Development of orodispersible polymer films containing poorly water soluble active pharmaceutical ingredients with focus on different drug loadings and storage stability. Int. J. Pharm. 2015, 493, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Amelian, A.; Szymańska, E.; Winnicka, K. Formulation and characterization of loratadine containing orodispersible lyophilizates and films. Acta Pol. Pharm. Drug Res. 2017, 74, 1533–1541. [Google Scholar]
- Juluru, N.S. Fast dissolving oral films: A review. Int. J. Adv. Pharm. Biol. Chem. 2013, 2, 108–112. [Google Scholar]
- Centkowska, K.; Ławrecka, E.; Sznitowska, M. Technology of orodispersible polymer films with micronized loratadine—influence of different drug loadings on film properties. Pharmaceutics 2020, 12, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łyszczarz, E.; Brniak, W.; Szafraniec-Szczęsny, J.; Majka, T.M.; Majda, D.; Zych, M.; Pielichowski, K.; Jachowicz, R. The impact of the preparation method on the properties of orodispersible films with aripiprazole: Electrospinning vs. casting and 3D printing methods. Pharmaceutics 2021, 13, 1122. [Google Scholar] [CrossRef] [PubMed]
- Cupone, I.E.; Dellera, E.; Marra, F.; Giori, A.M. Development and characterization of an orodispersible film for vitamin D3 supplementation. Molecules 2020, 25, 5851. [Google Scholar] [CrossRef] [PubMed]
- Callahan, J.C.; Cleary, G.W.; Elefant, M.; Kaplan, G.; Kensler, T.; Nash, R.A. Equilibrium moisture content of pharmaceutical excipients. Drug Dev. Ind Pharm. 1982, 8, 355–369. [Google Scholar] [CrossRef]
- Ward, I.M.; Sweeney, J. Mechanical properties of solid polymers, 3th ed.; John Wiley & Sons, Ltd: Hobokoen, NJ, USA, 2012. [Google Scholar]
- Brniak, W.; Maślak, E.; Jachowicz, R. Orodispersible films and tablets with prednisolone microparticles. Eur. J. Pharm. Sci. 2015, 75, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Henríquez, L.C.; Redondo, G.M.; Zúñiga, R.V.; Berrocal, G.C. Identification of rupatadine fumarate polymorphic crystalline forms in pharmaceutical raw materials. AJST 2018, 9, 7482–7487. [Google Scholar]
- Henríquez, L.C.; Zúñiga, R.V.; Redondo, G.M.; Berrocal, G.C.; Vargas, G.H. Determination of the impact caused by direct compression on the crystalline state of rupatadine fumarate 10 mg tablets. Int. J. Pharm. Technol. Biotechnol. 2019, 6, 1–12. [Google Scholar]
- Lai, H.L.; Pitt, K.; Craiga, D.Q.M. Characterisation of the thermal properties of ethylcellulose using differential scanning and quasi-isothermal calorimetric approaches. Int. J. Pharm. 2010, 386, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, K.; Ciosek-Skibińska, P.; Lenik, J.; Srcic, S.; Basa, A.; Winnicka, K. Utilization of ethylcellulose microparticles with rupatadine fumarate in designing orodispersible minitablets with taste masking effect. Materials 2020, 13, 2715. [Google Scholar] [CrossRef] [PubMed]
- Pein, M.; Preis, M.; Eckert, C.; Kiene, F.E. Taste-masking assessment of solid oral dosage forms—a critical review. Int. J. Pharm. 2014, 465, 239–254. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Eudragit: A novel carrier for controlled drug delivery in supercritical antisolvent coprecipitation. Polymers 2020, 12, 234. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, R.; Aleih, H.; Mashaqbeh, H.; Altaani, B.; Alsmadi, M.M.; Alnaief, M. Development and evaluation of cocoa butter taste masked ibuprofen using supercritical carbon dioxide. AAPS PharmSciTech 2021, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; De los Santos, D.M.; Martínez de la Ossa, E.J. Ibuprofen–polymer precipitation using supercritical CO2 at low temperature. J. Supercrit. Fluids 2014, 94, 91–101. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, A.H.; Soto, J.; Ernest, T.; Tuleu, C. Non-human tools for the evaluation of bitter taste in the design and development of medicines: A systematic review. Drug Discov. Today 2016, 21, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.; Kirsanov, D.; Legin, A.; Rudnitskaya, A.; Saunders, K. Assessing taste without using humans: Rat brief access aversion model and electronic tongue. Int. J. Pharm. 2012, 435, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Legin, A.; Rudnitskaya, A.; Clapham, D.; Seleznev, B.; Lord, K.; Vlasov, Y. Electronic tongue for pharmaceutical analytics: Quantification of tastes and masking effects. Anal. Bioanal. Chem. 2004, 380, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Williams, L.J.; McIntosh, A.R.; Abdi, H. Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. NeuroImage 2011, 56, 455–475. [Google Scholar] [CrossRef] [PubMed]
- Wesoły, M.; Ciosek-Skibińska, P. Comparison of performance of various data analysis techniques applied for the classification of pharmaceutical samples by electronic tongue. Sens. Actuators B Chem. 2018, 267, 570–580. [Google Scholar] [CrossRef]
- Zabadaj, M.; Szuplewska, A.; Kalinowska, D.; Chudy, M.; Ciosek-Skibińska, P. Studying pharmacodynamic effects in cell cultures by chemical fingerprinting - SIA electronic tongue versus 2D fluorescence soft sensor. Sens. Actuators B Chem. 2018, 272, 264–273. [Google Scholar] [CrossRef]
- Zabadaj, M.; Ufnalska, I.; Chreptowicz, K.; Mierzejewska, J.; Wróblewski, W.; Ciosek-Skibińska, P. Performance of hybrid electronic tongue and HPLC coupled with chemometric analysis for the monitoring of yeast biotransformations. Chehometr. Intell. Lab. Systs. 2017, 167, 69–77. [Google Scholar] [CrossRef]






| Ingredient (%) | Formulation | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| RUP | - | 0.25 | - | - |
| MP-S-RUP | - | - | 0.51 | - |
| MP-A-RUP | - | - | - | 0.62 |
| HPMC | 12.0 | 12.0 | 12.0 | 12.0 |
| Glycerol | 6.0 | 6.0 | 6.0 | 6.0 |
| Purifiedwater | 82.0 | 81.75 | 81.49 | 81.38 |
| El. no. | Electrode Type | Ionophore (% w/w) | Lipophilic Salt (% w/w) | Plasticiser (% w/w) | Polymer (% w/w) |
|---|---|---|---|---|---|
| 1–2 | CS-D | - | KTpCPB (3%) | DOS (65%) | PVC (32%) |
| 3–4 | CS-N | - | KTpCPB (3%) | o-NPOE (65%) | PVC (32%) |
| 5–6 | CS-AN-D | - | TDMA-TCPB (3%) | DOS (65%) | PVC (32%) |
| 7–8 | CS-AN-N | - | TDMA-TCPB (3%) | o-NPOE (65%) | PVC (32%) |
| 9–10 | AN-D | TDAC (3.5%) | DOS (64%) | PVC (32.5%) | |
| 11–12 | AN- N | - | TDAC (3.5%) | o-NPOE (64%) | PVC (32.5%) |
| 13−14 | AM-D | amine ionophore I (5%) | - | DOS (68%) | PVC (27%) |
| 15–16 | NI-N | - | TDMAN (6%) | o-NPOE (62%) | PVC (32%) |
| Formulation | Thickness [µm] | Weight [mg] | Moisture Content [%] | RUP Content [%] | Disintegration Time [s] | ||
|---|---|---|---|---|---|---|---|
| In Vivo | Petri Dish | Drop Method | |||||
| F1 | 131.03 ± 0.82 a | 16.05 ± 0.13 a | 6.21 ± 0.47 a | not applicable | 18.00 ± 0.82 a | 17.21 ± 0.19 a | 22.50 ± 0.58 a |
| F2 | 133.21 ± 0.49 b | 18.03 ± 0.13 b | 3.74 ± 0.29 b | 99.23 ± 2.55 a | 18.75 ± 0.50 ac | 18.34 ± 0.26 b | 24.00 ± 0.82 b |
| F3 | 133.92 ± 1.27 b | 20.03 ± 0.17 c | 3.92 ± 0.18 b | 93.26 ± 1.72 b | 20.25 ± 0.96 bc | 19.18 ± 0.13 c | 24.75 ± 0.50 b |
| F4 | 134.91 ± 0.82 b | 21.43 ± 0.46 d | 3.55 ± 0.37 b | 101.29 ± 2.26 a | 21.25 ± 0.96 bd | 20.13 ± 0.18 d | 25.0 ± 0.82 b |
| Formulation | Tear Resistance [N] | Tensile Strength [N/mm2] | Elongation at Break [%] | Young’s Modulus [MPa] | Folding Endurance |
|---|---|---|---|---|---|
| F1 | 18.35 ± 0.27 a | 7.12 ± 0.06 a | 2.74 ± 0.03 a | 611.1.1 ± 0.45 a | >300 |
| F2 | 17.31 ± 0.30 bc | 6.41 ± 0.10 b | 1.52 ± 0.04 b | 792.3 ± 0.73 b | ≤100 |
| F3 | 17.86 ± 0.33 ac | 6.61 ± 0.05 b | 2.18 ± 0.06 c | 692.4 ± 0.66 c | ≤100 |
| F4 | 15.49 ± 0.42 d | 5.94 ± 0.15 c | 1.37 ± 0.04 d | 966.3 ± 0.19 d | ≤100 |
| Volunteer/ Formulation | Score | ||
|---|---|---|---|
| F2 | F3 | F4 | |
| A | 3 | 1 | 1 |
| B | 3 | 2 | 1 |
| C | 3 | 0 | 0 |
| D | 3 | 1 | 1 |
| E | 3 | 1 | 0 |
| F | 3 | 1 | 0 |
| Lp | Electrode Type | Sensitivity ± s, mV/Decade | Linear Range −log c, mol/L | R ± SD |
|---|---|---|---|---|
| 1 | CS-D | 4.1 ± 6.3 | 3–5 | 0.9980 ± 0.0035 |
| 2 | CS-N | 53.5 ± 10.8 | 3–5 | 0.9997 ± 0.0003 |
| 3 | CS-AN-D | 24.8 ± 0.6 | 3–6 | 0.9884 ± 0.0034 |
| 4 | CS-AN-N | 31.4 ± 2.3 | 3–6 | 0.9720 ± 0.0090 |
| 5 | AN-D | −19.5 ± 2.0 | 4–6 | 0.9897 ± 0.0150 |
| 6 | AN-N | −22.4 ± 2.9 | 4–6 | 0.9919 ± 0.0063 |
| 7 | AM-D | 40.4 ± 5.0 | 3–5 | 0.9981 ± 0.0015 |
| 8 | NI-N | −22.8 ± 3.2 | 4–6 | 0.9936 ± 0.0079 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olechno, K.; Maciejewski, B.; Głowacz, K.; Lenik, J.; Ciosek-Skibińska, P.; Basa, A.; Winnicka, K. Orodispersible Films with Rupatadine Fumarate Enclosed in Ethylcellulose Microparticles as Drug Delivery Platform with Taste-Masking Effect. Materials 2022, 15, 2126. https://doi.org/10.3390/ma15062126
Olechno K, Maciejewski B, Głowacz K, Lenik J, Ciosek-Skibińska P, Basa A, Winnicka K. Orodispersible Films with Rupatadine Fumarate Enclosed in Ethylcellulose Microparticles as Drug Delivery Platform with Taste-Masking Effect. Materials. 2022; 15(6):2126. https://doi.org/10.3390/ma15062126
Chicago/Turabian StyleOlechno, Katarzyna, Bartosz Maciejewski, Klaudia Głowacz, Joanna Lenik, Patrycja Ciosek-Skibińska, Anna Basa, and Katarzyna Winnicka. 2022. "Orodispersible Films with Rupatadine Fumarate Enclosed in Ethylcellulose Microparticles as Drug Delivery Platform with Taste-Masking Effect" Materials 15, no. 6: 2126. https://doi.org/10.3390/ma15062126
APA StyleOlechno, K., Maciejewski, B., Głowacz, K., Lenik, J., Ciosek-Skibińska, P., Basa, A., & Winnicka, K. (2022). Orodispersible Films with Rupatadine Fumarate Enclosed in Ethylcellulose Microparticles as Drug Delivery Platform with Taste-Masking Effect. Materials, 15(6), 2126. https://doi.org/10.3390/ma15062126