Two-Dimensional Cs3Sb2I9−xClx Film with (201) Preferred Orientation for Efficient Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Device Fabrication
2.3. Device Characterization
2.4. DFT Calculations
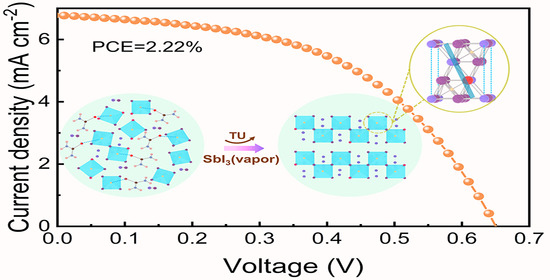
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NREL. Best Research-Cell Efficiencies. Available online: https://www.nrel.gov/pv/device-performance.html (accessed on 3 December 2021).
- Tumen-Ulzii, G.; Qin, C.; Klotz, D.; Leyden, M.R.; Wang, P.; Auffray, M.; Fujihara, T.; Matsushima, T.; Lee, J.W.; Lee, S.J. Detrimental effect of unreacted PbI2 on the long-term stability of perovskite solar cells. Adv. Mater. 2020, 32, 1905035. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yu, B.-B.; Liao, M.; Liu, D.; Xiu, J.; Zhang, Z.; Lifshitz, E.; Tang, J.; Song, H.; He, Z. Enhanced efficiency and stability in Sn-based perovskite solar cells with secondary crystallization growth. J. Energy Chem. 2021, 54, 414–421. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Li, L.; Tu, Z.; Song, L.; Ding, B.; Wang, R.; Xu, Y. Density functional theory analysis of structural and electronic properties of hexagonal hybrid perovskite (CH3NH3)3Bi2I9. Phys. B Condens. Matter 2022, 630, 413695. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Zhou, Q.; Wei, Q.; Wei, M.; Jiang, L.; Wang, Z.; Peng, Z.; Wang, F.; Zang, Z. One-step synthesis of SnI2·(DMSO)x adducts for high-performance tin perovskite solar cells. J. Am. Chem. Soc. 2021, 143, 10970–10976. [Google Scholar] [CrossRef]
- Jain, S.M.; Phuyal, D.; Davies, M.L.; Li, M.; Philippe, B.; de Castro, C.; Qiu, Z.; Kim, J.; Watson, T.; Tsoi, W.C. An effective approach of vapour assisted morphological tailoring for reducing metal defect sites in lead-free, (CH3NH3)3Bi2I9 bismuth-based perovskite solar cells for improved performance and long-term stability. Nano Energy 2018, 49, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, C.; Cai, M.; Liao, Y.; Ding, Y.; Ma, S.; Liu, X.; Guli, M.; Dai, S.; Nazeeruddin, M.K. Dimension-controlled growth of antimony-based perovskite-like halides for lead-free and semitransparent photovoltaics. ACS. Appl. Mater. Interfaces 2020, 12, 17062–17069. [Google Scholar] [CrossRef]
- Schileo, G.; Grancini, G. Lead or no lead? Availability, toxicity, sustainability and environmental impact of lead-free perovskite solar cells. J. Mater. Chem. C 2021, 9, 67–76. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, D.; Jiang, Y.; Liu, T.; Zhao, X.; Ming, Y.; Luo, B.; Qin, F.; Fan, J.; Han, H. Chlorine-incorporation-induced formation of the layered phase for antimony-based lead-free perovskite solar cells. J. Am. Chem. Soc. 2018, 140, 1019–1027. [Google Scholar] [CrossRef]
- Singh, A.; Lai, P.-T.; Mohapatra, A.; Chen, C.-Y.; Lin, H.-W.; Lu, Y.-J.; Chu, C.W. Panchromatic heterojunction solar cells for Pb-free all-inorganic antimony based perovskite. Chem. Eng. J. 2021, 419, 129424. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, F.; Shen, J.; Zhou, Y.; Wu, Y.; Guo, Y.; Jiang, J.; Chu, J. Inorganic lead-free antimony-based perovskite-inspired solar cells with a carbon electrode and green anti-solvent regulation. J. Mater. Chem. 2021, 9, 15301–15308. [Google Scholar] [CrossRef]
- Pal, J.; Manna, S.; Mondal, A.; Das, S.; Adarsh, K.; Nag, A. Colloidal Synthesis and Photophysics of M3Sb2I9 (M = Cs and Rb) Nanocrystals: Lead-Free Perovskites. Angew. Chem. Int. Ed. 2017, 56, 14187–14191. [Google Scholar] [CrossRef] [PubMed]
- Umar, F.; Zhang, J.; Jin, Z.; Muhammad, I.; Yang, X.; Deng, H.; Jahangeer, K.; Hu, Q.; Song, H.; Tang, J. Dimensionality Controlling of Cs3Sb2I9 for Efficient All-Inorganic Planar Thin Film Solar Cells by HCl-Assisted Solution Method. Adv. Opt. Mater. 2019, 7, 1801368. [Google Scholar] [CrossRef]
- Peng, Y.; Li, F.; Wang, Y.; Li, Y.; Hoye, R.L.; Feng, L.; Xia, K.; Pecunia, V. Enhanced photoconversion efficiency in cesium-antimony-halide perovskite derivatives by tuning crystallographic dimensionality. Appl. Mater. Today 2020, 19, 100637. [Google Scholar] [CrossRef]
- Liang, P.W.; Liao, C.Y.; Chueh, C.C.; Zuo, F.; Williams, S.T.; Xin, X.K.; Lin, J.; Jen, A.K. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 2014, 26, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Najman, S.; Mohapatra, A.; Lu, Y.-J.; Hanmandlu, C.; Pao, C.-W.; Chen, Y.-F.; Lai, C.S.; Chu, C.-W. Modulating performance and stability of inorganic lead-free perovskite solar cells via lewis-pair mediation. ACS. Appl. Mater. Interfaces 2020, 12, 32649–32657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, T.; Kaplan, A.B.; Yao, C.; Loo, Y.-L. Accessing highly oriented two-dimensional perovskite films via solvent-vapor annealing for efficient and stable solar cells. Nano Lett. 2020, 20, 8880–8889. [Google Scholar] [CrossRef]
- Donnay, J.D.H.; Harker, D. A new law of crystal morphology extending the law of Bravais. Am. Mineral. J. Earth Planet. Mater. 1937, 22, 446–467. [Google Scholar]
- Li, J.; Liu, X.; Yao, J. The enhanced photovoltaic performance of Sb2S3 solar cells by thermal decomposition of antimony ethyl xanthate with thiourea doping. Energy Technol. 2020, 8, 1900841. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Gregory, N.W. Equilibrium vapor concentrations in the antimony+ iodine system. Molar absorptivities of antimony (III) iodide vapor. J. Chem. Eng. Data 1996, 41, 107–112. [Google Scholar] [CrossRef]
- Singh, A.; Boopathi, K.M.; Mohapatra, A.; Chen, Y.F.; Li, G.; Chu, C.W. Photovoltaic performance of vapor-assisted solution-processed layer polymorph of Cs3Sb2I9. ACS. Appl. Mater. Interfaces 2018, 10, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Pal, A.J.; Larson, B.W. Structure, Morphology, and Photovoltaic Implications of Halide Alloying in Lead-Free Cs3Sb2ClxI9–x 2D-Layered Perovskites. Sol. RRL 2021, 5, 2000422. [Google Scholar] [CrossRef]
- Chelvanathan, P.; Yusoff, Y.; Haque, F.; Akhtaruzzaman, M.; Alam, M.; Alothman, Z.; Rashid, M.; Sopian, K.; Amin, N. Growth and characterization of RF-sputtered ZnS thin film deposited at various substrate temperatures for photovoltaic application. Appl. Surf. Sci. 2015, 334, 138–144. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Hong, C.K. A thiourea additive-based quadruple cation lead halide perovskite with an ultra-large grain size for efficient perovskite solar cells. Nanoscale 2019, 11, 21824–21833. [Google Scholar] [CrossRef]
- Li, X.; He, B.; Gong, Z.; Zhu, J.; Zhang, W.; Chen, H.; Duan, Y.; Tang, Q. Compositional engineering of Chloride Ion-Doped CsPbBr3 halides for highly efficient and stable all-inorganic perovskite solar cells. Sol. RRL 2020, 4, 2000362. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Chen, H.; Ji, L.; Zheng, H.; Gu, Y.; Wang, F.; Chen, Z.; Li, S. Enhanced Crystallinity of Triple-Cation Perovskite Film via Doping NH4SCN. Nanoscale Res. Lett. 2019, 14, 304. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Luo, H.; Hu, J.; Li, H.; Gao, P. Efficient Perovskite Solar Cells by Reducing Interface-Mediated Recombination: A Bulky Amine Approach. Adv. Energy Mater. 2020, 10, 2000197. [Google Scholar] [CrossRef]
- Wang, R.; Xue, J.; Wang, K.-L.; Wang, Z.-K.; Luo, Y.; Fenning, D.; Xu, G.; Nuryyeva, S.; Huang, T.; Zhao, Y. Constructive molecular conFigureurations for surface-defect passivation of perovskite photovoltaics. Science 2019, 366, 1509–1513. [Google Scholar] [CrossRef]
- Tiwari, D.; Fermin, D.J.; Chaudhuri, T.; Ray, A. Solution processed bismuth ferrite thin films for all-oxide solar photovoltaics. J. Phys. Chem. C 2015, 119, 5872–5877. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Z.; Zhang, S.; Si, H.; Ma, S.; Fan, W.; Xiong, Z.; Liao, Q.; Sattar, A.; Kang, Z. Manipulation of Perovskite Crystallization Kinetics via Lewis Base Additives. Adv. Funct. Mater. 2021, 31, 2009425. [Google Scholar] [CrossRef]
- Gibbs, J.W. On the equilibrium of heterogeneous substances. Am. J. Sci. 1879, 3, 441–458. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, X.; Chen, B.; Kelly, L.L.; Zhao, J.; Lin, Y.; Toney, M.F.; Huang, J.S. Crystallization in one-step solution deposition of perovskite films: Upward or downward? Sci. Adv. 2021, 7, eabb2412. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef] [Green Version]
- Cao, K.; Cheng, Y.; Chen, J.; Huang, Y.; Ge, M.; Qian, J.; Liu, L.; Feng, J.; Huang, W. Regulated Crystallization of FASnI3 Films through Seeded Growth Process for Efficient Tin Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 41454–41463. [Google Scholar] [CrossRef] [PubMed]
- Bube, R.H. Trap density determination by space-charge-limited currents. J. Appl. Phys. 1962, 33, 1733–1737K. [Google Scholar] [CrossRef]
- Wang, Y.; Dar, M.I.; Ono, L.K.; Zhang, T.; Kan, M.; Li, Y.; Zhang, L.; Wang, X.; Yang, Y.; Gao, X. Thermodynamically stabilized β-CsPbI3–based perovskite solar cells with efficiencies > 18%. Science 2019, 365, 591–595. [Google Scholar] [CrossRef]
- You, P.; Tang, G.; Cao, J.; Shen, D.; Ng, T.-W.; Hawash, Z.; Wang, N.; Liu, C.-K.; Lu, W.; Tai, Q.; et al. Applications, 2D materials for conducting holes from grain boundaries in perovskite solar cells. Light Sci. Appl. 2021, 10, 68. [Google Scholar] [CrossRef]
- Hiltunen, A.; Lamminen, N.; Salonen, H.; Liu, M.; Vivo, P. Efficiency improvement for perovskite-inspired Cs3Sb2I9 solar cells using P3HT as the hole transport material. Sustain. Energy Fuels 2022, 6, 217–222. [Google Scholar] [CrossRef]
- Bai, D.; Zhang, J.; Jin, Z.; Bian, H.; Wang, K.; Wang, H.; Liang, L.; Wang, Q.; Liu, S.F. Interstitial Mn2+-driven high-aspect-ratio grain growth for low-trap-density microcrystalline films for record efficiency CsPbI2Br solar cells. ACS Energy Lett. 2018, 3, 970–978. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lv, Y.; Han, H.; Xu, J.; Yao, J. Two-Dimensional Cs3Sb2I9−xClx Film with (201) Preferred Orientation for Efficient Perovskite Solar Cells. Materials 2022, 15, 2883. https://doi.org/10.3390/ma15082883
Li J, Lv Y, Han H, Xu J, Yao J. Two-Dimensional Cs3Sb2I9−xClx Film with (201) Preferred Orientation for Efficient Perovskite Solar Cells. Materials. 2022; 15(8):2883. https://doi.org/10.3390/ma15082883
Chicago/Turabian StyleLi, Jihong, Yongao Lv, Huifang Han, Jia Xu, and Jianxi Yao. 2022. "Two-Dimensional Cs3Sb2I9−xClx Film with (201) Preferred Orientation for Efficient Perovskite Solar Cells" Materials 15, no. 8: 2883. https://doi.org/10.3390/ma15082883
APA StyleLi, J., Lv, Y., Han, H., Xu, J., & Yao, J. (2022). Two-Dimensional Cs3Sb2I9−xClx Film with (201) Preferred Orientation for Efficient Perovskite Solar Cells. Materials, 15(8), 2883. https://doi.org/10.3390/ma15082883