Development of Low Shrinkage Curing Techniques for Unsaturated Polyester and Vinyl Ester Reinforced Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Preparation of Dumbbell-Shaped Mold for Reinforce Composite
2.1.2. Characterization of UPE and VE Reinforced Matrix Cured under Air and CO2
2.1.3. DSC Evaluation
2.1.4. Tensile Strength
2.1.5. Flexural Strength Test
2.1.6. Flexural Modulus Assessment
2.1.7. Contact Angle
3. Results and Discussion
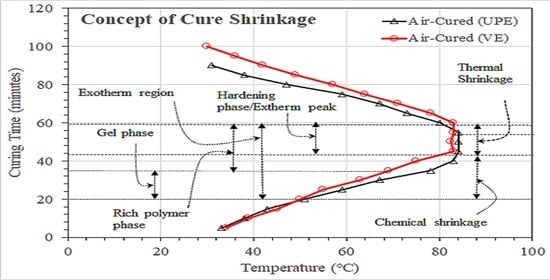
3.1. Molding and Cured Shrinkage Analysis
3.1.1. Effect of Air and CO2 Curing on Resin Gel Time and Exotherm
3.1.2. Differential Scanning Calorimetry (DSC) Analysis
3.1.3. Thermal Stability
3.1.4. Contact Angle and Water Absorption Comparison of Air- and CO2-Cured Specimens
4. Mechanical Properties
Tensile Strength of UPE and VE
Flexural Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ardhyananta, H.; Puspadewa, F.D.; Wicaksono, S.T.; Widyastuti; Wibisono, A.T.; Kurniawan, B.A.; Ismail, H.; Salsac, A.V. Mechanical and Thermal Properties of Unsaturated Polyester/Vinyl Ester Blends Cured at Room Temperature. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012088. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q. Thermosets: Structure, Properties, and Applications, Part 2 Application of Thermoset, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Nawab, Y.; Tardif, X.; Boyard, N.; Sobotka, V.; Casari, P.; Jacquemin, F. Determination and modelling of the cure shrinkage of epoxy vinylester resin and associated composites by considering thermal gradients. Compos. Sci. Technol. 2012, 73, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Abou Msallem, Y.; Jacquemin, F.; Boyard, N.; Poitou, A.; Delaunay, D.; Chatel, S. Material characterization and residual stresses simulation during the manufacturing process of epoxy matrix composites. Compos. Part. A Appl. Sci. Manuf. 2010, 41, 108–115. [Google Scholar] [CrossRef]
- Shimbo, M.; Yamabe, M.; Miyano, Y. Viscoelastic analysis of residual stress in thermosetting resins introduced during curing process. Exp. Mech. 1998, 2, 1325–1330. [Google Scholar]
- Nawab, Y.; Jacquemin, F.; Casari, P.; Boyard, N.; Sobotka, V. Evolution of chemical and thermal curvatures in thermoset laminated composite plates during the fabrication process. J. Compos. Mater. 2012, 47, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Rachmadini, Y.; Tan, V.B.C.; Tay, T.E. Enhancement of Mechanical Properties of Composites through Incorporation of CNT in VARTM A Review. J. Reinf. Plast. Compos. 2010, 29, 18. [Google Scholar] [CrossRef]
- Merle, G.; Pascault, J.P. Influence of Processing Conditions on Shrinkage Behavior of Low-Profile Unsaturated Polyester Resins. I: Systems without Fillers. Polym. Eng. Sci. 1993, 33, 466–473. [Google Scholar]
- Kravchenko, O.G.; Kravchenko, S.G.; Pipes, R.B. Chemical and thermal shrinkage in thermosetting prepreg. Composites Part A Appl. Sci. Manuf. 2016, 80, 72–81. [Google Scholar] [CrossRef]
- Schoch, K.F.; Panackal, P.A.; Frank, P.P. Real-time measurement of resin shrinkage during cure. Thermochim. Acta 2004, 417, 115–118. [Google Scholar] [CrossRef]
- Li, C.; Potter, K.; Wisnom, M.R.; Stringer, G. In-situ measurement of chemical shrinkage of MY750 epoxy resin by a novel gravimetric method. Compos. Sci. Technol. 2004, 64, 55–64. [Google Scholar] [CrossRef]
- Parlevliet, P.P.; Bersee, H.E.N.; Beukers, A. Shrinkage determination of a reactive polymer with volumetric dilatometry. Polym. Test. 2010, 29, 433–439. [Google Scholar] [CrossRef]
- Parlevliet, P.P.; Bersee, H.E.N.; Beukers, A. Measurement of (post-)curing strain development with fibre Bragg gratings. Polym. Test. 2010, 29, 291–301. [Google Scholar] [CrossRef]
- Antonucci, V.; Giordano, M.; Cusano, A.; Nasser, J.; Nicolais, L. Real time monitoring of cure and gelification of a thermoset matrix. Compos. Sci. Technol. 2006, 66, 3273–3280. [Google Scholar] [CrossRef]
- Karalekas, D.; Cugnoni, J.; Botsis, J. Monitoring of process induced strains in a single fibre composite using FBG sensor: A methodological study. Compos. A Appl. Sci. Manuf. 2008, 39, 1118–1127. [Google Scholar] [CrossRef]
- Giordano, M.; Laudati, A.; Nasser, J.; Nicolais, L.; Cusano, A.; Cutolo, A. Monitoring by a single fiber Bragg grating of the process induced chemo-physical transformations of a model thermoset. Sens. Actuators A 2004, 113, 166–173. [Google Scholar] [CrossRef]
- Vacher, S.; Molimard, J.; Gagnaire, H.; Vautrin, A. A fresnel’s reflection optical fiber sensor for thermoset polymer cure monitoring. Polym. Polym. Compos. 2003, 12, 269–276. [Google Scholar] [CrossRef]
- Snow, A.W.; Armistead, J.P. A simple dilatometer for thermoset cure shrinkage and thermal expansion measurements. J. Appl. Polym. Sci. 1994, 52, 401–411. [Google Scholar] [CrossRef]
- Jyi, Y.H.; Ming, C.L. Volume shrinkage characteristics in the cure of lowshrink unsaturated polyester resins. Polymer 1996, 37, 401–412. [Google Scholar]
- Mark, K.; Lee, L.J. Development of a dilatometer and its application to low-shrink unsaturated polyester resins. J. Appl. Polym. Sci. 1992, 45, 37–50. [Google Scholar]
- Mark, K.; Shailesh, M.; Lee, L.J. Dilatometric study of low profile unsaturated polyester resins. Polym. Eng. Sci. 1995, 35, 823–836. [Google Scholar]
- Madhukar, M.S.; Genidy, M.S.; Russell, J.D. A new method to reduce cure-induced stresses in thermoset polymer composites, Part I: Test method. J. Compos. Mater. 2000, 34, 1882–1904. [Google Scholar] [CrossRef]
- Tai, H.J.; Chou, H.L. Chemical shrinkage and diffusion-controlled reaction of an epoxy molding compound. Eur. Polym. J. 2000, 36, 2213–2219. [Google Scholar] [CrossRef]
- Lange, J.; Toll, S.; Månson, A.E.; Hult, A. Residual stress build-up in thermoset films cured above their ultimate glass transition temperature. Polymer 1995, 36, 3135–3141. [Google Scholar] [CrossRef]
- Yang, D.G.; Jansen, K.M.B.; Ernst, L.J.; Zhang, G.Q.; Bressers, H.J.L.; Janssen, J.H.J. Effect of filler concentration of rubbery shear and bulk modulus of molding compounds. Microelectron. Reliab. 2007, 47, 233–239. [Google Scholar] [CrossRef]
- Hoa, S.V.; Ouellette, P.; Ngo, T.D. Determination of shrinkage and modulus development of thermosetting resins. J. Compos. Mater. 2009, 43, 783–803. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.S.; Lee, L.J. Microstructure formation in the cure of unsaturated polyester resins. Polymer 1988, 29, 1793–1800. [Google Scholar] [CrossRef]
- Cadenato, A.; Salla, J.M.; Ramis, X.; Morancho, J.M.; Marroyo, L.M.; Martin, J.L. determination of gel and vitrification times of thermoset curing process by means of TMA, DMTA and DSC techniques TTT diagram. J. Therm. Anal. 1997, 49, 269–279. [Google Scholar] [CrossRef]
- Sakaguchi1, R.L.; Versluis, A.; Douglas, W.H. Analysis of strain gage method for measurement of post-gel shrinkage in resin composites. Dent. Mater 1997, 13, 233–239. [Google Scholar] [CrossRef]
- Shah, D.U.; Schubel, P.J. Evaluation of cure shrinkage measurement techniques for thermosetting resins. Polym. Test. 2010, 29, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Sadeghinia, M.; Jansen, K.M.B.; Ernst, L.J. Characterization and modeling the thermo-mechanical cure-dependent properties of epoxy molding compound. Int. J. Adhes. 2012, 32, 82–88. [Google Scholar] [CrossRef]
- Fernandez-Francos, X.; Kazarian, S.G.; Ramis, X.; Serra, A. Simultaneous monitoring of curing shrinkage and degree of cure of thermosets by Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopy. Appl. Spectrosc. 2013, 67, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Oota, K.; Saka, M. Cure Shrinkage Analysis of Epoxy Molding Compound. Polym. Eng. Sci. 2001, 41, 1373–1379. [Google Scholar] [CrossRef]
- McClory, C.; McNally, T.; Brennan, G.P.; Erskine, J. Thermosetting Polyurethane Multiwalled Carbon Nanotube Composites. J. Appl. Polym. Sci. 2007, 105, 1003–1011. [Google Scholar] [CrossRef]
- Sua, Y.; Zhanga, S.; Zhang, Z.; Zhao, X.; Jing, D. Preparation and properties of carbon nanotubes/carbon fiber/poly (ether ketone) multiscale composites. Compos. Part A 2008, 108, 89–98. [Google Scholar] [CrossRef]
- Hong, C.M.; Wang, X.; Kong, P.; Pan, Z.; Wang, X. Effect of succinic acid on the shrinkage of unsaturated polyester resin. J. Appl. Polym. Sci. 2015, 132, 2. [Google Scholar] [CrossRef]
- Kinkelaar, M.; Wang, B.; Lee, L.J. Shrinkage behaviour of low-profile unsaturated polyester resins. Polymer 1994, 35, 3011–3022. [Google Scholar] [CrossRef]
- Cao, X.; Lee, L.J. Control of shrinkage and residual styrene of unsaturated polyester resins cured at low temperatures: I. Effect of curing agents. Polymer 2003, 44, 1893–1902. [Google Scholar] [CrossRef]
- Voto, G.; Sequeira, L.; Skordos, A.A. Formulation based predictive cure kinetics modelling of epoxy resins. Polymer 2021, 236, 124304. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Y.; Zhang, Y. A Study on the Synthesis, Curing Behavior and Flame Retardance of a Novel Flame Retardant Curing Agent for Epoxy Resin. Polymers 2022, 14, 245. [Google Scholar] [CrossRef]
- Wang, Z.; Pitchaimari, G.; Nair, S.S.; Yi, S.; Yan, N.; Daniels, J.H. Curing Behavior and Thermomechanical Performance of Bioepoxy Resin Synthesized from Vanillyl Alcohol: Effects of the Curing Agent. Polymers 2021, 13, 2891. [Google Scholar] [CrossRef]
- Sousa, G.S.; Guimaraes, G.F.; Marcelino, E.; Rodoka, J.E.O.; Junior, A.J.O.; Cesarino, I.; Leao, A.L.; Riccardi, C.S.; Arjmad, M.; Simoes, R.P. Shrinkage Stress and Temperature Variation in Resin Composites Cured via Different Photoactivation Methods: Insights for Standardisation of the Photopolymerisation. Polymers 2021, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Dodiuk, H.; Goodman, S.H. Handbook of Thermoset Plastics, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 6. [Google Scholar]
- Dirand, X.; Hilaire, B.; Lafontaine, E.; Mortaigne, B.; Nardin, M. Crosslinking of vinyl ester matrix in contact with different surfaces. Composites 1994, 25, 645–652. [Google Scholar] [CrossRef]
- Desnoes, E.; Toubal, L.; Thibeault, D.; Bouazza, A.H.; Montplaisir, D. Bio-sourced vinyl ester resin reinforced with microfibrillar cellulose: Mechanical and thermal properties. Polym. Polym. Compos. 2021, 29, 306–316. [Google Scholar] [CrossRef]
- Goswami, S.; Ranjan, J.K. Study of Mechanical and Thermomechanical Properties of Vinyl Ester/Polyurethane Interpenetrating Polymer Network Based Hybrid Composites. Fibers Polym. 2020, 21, 1096–1114. [Google Scholar] [CrossRef]
- Abral, H.; Kadriadi, D.; Rodianus, A.; Mastariyanto, P.; Ilhamdi; Arief, S.; Sapuan, S.M.; Ishak, M.R. Mechanical properties of water hyacinth fibers—Polyester composites before and after immersion in water. Mater. Des. 2014, 58, 125–129. [Google Scholar] [CrossRef]
- Rose, N.; Bras, M.L.; Delobel, R.; Costes, B.; Henry, Y. Thermal oxidative degradation of an epoxy resin. Polym. Degrad. Stab. 1993, 42, 307–316. [Google Scholar] [CrossRef]
- Safarabadi, M.; Shokrieh, M.M. Understanding Residual Stresses in Polymer Matrix Composites; Woodhead Publishing Limited: Cambridge, UK, 2014; Chapter 8. [Google Scholar]
- Shokrieh, M.; Ghanei, M.A.R. The Importance of Measuring Residual Stresses in Composite Materials. In Residual Stresses in Composite Materials; Shokrieh, M., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 3–14. [Google Scholar]












| Composition | A | B | C | D | E |
|---|---|---|---|---|---|
| Proportion (vol.%) | |||||
| MEKP | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| UPE and VE | 5 | 5 | 5 | 5 | 5 |
| Air | Ambient condition | ||||
| CO2 | 99.99% | ||||
| MEKP (Conc.) | Air Cured UPE | CO2 Cured UPE | ||||
|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.5 | 0.1 | 0.3 | 0.5 | |
 |  |  |  |  |  | |
| Heat (J/g) | 2 | 0.893 | 1.932 | 2.4 | 1.126 | 2.334 |
| T (°C) | 57.4–103 | 49.2–75.5 | 30–68 | 57–84 | 40–80 | 33–69.7 |
| Time (min) | 9.3–18.4 | 7.6–12.9 | 4.4–11.4 | 9.1–16.5 | 6.8–14.2 | 5.7–11.6 |
| Peak Max (°C/min) | 69/11.6 | 60.8/9.9 | 38/5.4 | 68.3/12 | 56.4/10.2 | 37/6.1 |
| Peak Hight (mW) | −0.122 | −0.103 | −0.232 | −0.113 | −0.45 | −0.195 |
| Heat (J/g) | Air Cured VE | CO2 Cured VE | ||||
 |  |  |  |  |  | |
| 1.705 | 0.92 | 1.806 | 1.851 | 0.98 | 1.87 | |
| T (°C) | 62–101 | 28–66 | 46.4–72.2 | 65–110 | 29–73 | 51–80 |
| Time (min) | 10.3–18 | 3.7–10.9 | 7–12.2 | 10.9–19.5 | 4–13.1 | 8.1–14.7 |
| Peak Max (°C/min) | 80.1/13.8 | 33.4/4.6 | 60/9.7 | 80/15.8 | 36/5.7 | 60/13.8 |
| Peak Height (mW) | −0.101 | −0.068 | −0.52 | −0.105 | −0.108 | −0.104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moujdin, I.A.; Totah, H.S.; Abulkhair, H.A.; Alsaiari, A.O.; Shaiban, A.A.; Organji, H.A. Development of Low Shrinkage Curing Techniques for Unsaturated Polyester and Vinyl Ester Reinforced Composites. Materials 2022, 15, 2972. https://doi.org/10.3390/ma15092972
Moujdin IA, Totah HS, Abulkhair HA, Alsaiari AO, Shaiban AA, Organji HA. Development of Low Shrinkage Curing Techniques for Unsaturated Polyester and Vinyl Ester Reinforced Composites. Materials. 2022; 15(9):2972. https://doi.org/10.3390/ma15092972
Chicago/Turabian StyleMoujdin, Iqbal Ahmed, Husam Saber Totah, Hani Abdulelah Abulkhair, Abdulmohsen Omar Alsaiari, Amer Ahmed Shaiban, and Hussam Adnan Organji. 2022. "Development of Low Shrinkage Curing Techniques for Unsaturated Polyester and Vinyl Ester Reinforced Composites" Materials 15, no. 9: 2972. https://doi.org/10.3390/ma15092972
APA StyleMoujdin, I. A., Totah, H. S., Abulkhair, H. A., Alsaiari, A. O., Shaiban, A. A., & Organji, H. A. (2022). Development of Low Shrinkage Curing Techniques for Unsaturated Polyester and Vinyl Ester Reinforced Composites. Materials, 15(9), 2972. https://doi.org/10.3390/ma15092972