Fabrication of TiO2 ̶ KH550 ̶ PEG Super-Hydrophilic Coating on Glass Surface without UV/Plasma Treatment for Self-Cleaning and Anti-Fogging Applications
Abstract
:1. Introduction
2. Experimental Setup
2.1. Materials
2.2. Preparation of TiO2 Hydrophilic Film (TiO2 ̶ KH550 ̶ PEG ) and Deposition on Glass Substrate
2.2.1. Preparation of TiO2 ̶ KH550 ̶ PEG Hydrophilic Film
2.2.2. Deposition of TiO2-KH550-PEG Thin Film on Glass Substrate
Pretreatment
Deposition
2.3. Characterization of TiO2-KH550-PEG Thin-Film-Coated Glass Surface
2.3.1. X-ray Diffraction Analysis (XRD)
2.3.2. FT-IR Spectroscopy
2.3.3. Field Emission Scanning Electron Microscope (FESEM)
2.3.4. Water Contact Angle (WCA) Analysis and Optical Properties Measurement
2.3.5. Self-Cleaning Property Analysis and Adhesion Test
2.3.6. Anti-Fog Property Analysis
3. Results and Discussion
3.1. XRD Analysis
3.2. FT-IR Analysis
3.3. Surface Morphology Analysis
3.4. Wettability and Super-Hydrophilicity Property of the Coating
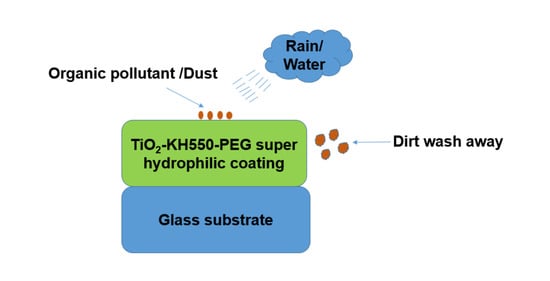
3.5. Self-Cleaning Test
3.6. Anti-Fogging Behavior of Coating and Stability of the Hydrophilicity in the Darkness
3.7. Transmittance Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.; Hakki, A.; Wang, F.; Donal, E.M. Photocatalyst efficiencies in concrete technology: The effect of photocatalyst placement. Appl. Catal. B 2018, 222, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Kako, T.; Meng, X.; Ye, J. Solid-base loaded WO3 photocatalyst for decomposition of harmful organics under visible light irradiation. APL Mater. 2015, 3, 104411. [Google Scholar] [CrossRef] [Green Version]
- Rabajczyk, A.; Zielecka, M.; Klapsa, W.; Dzechciarz, A. Self-cleaning coatings and surfaces of modern building materials for the removal of some air pollutant. Materials 2021, 14, 2161. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504526. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Kumar, A.M.; Sadasivuni, K.K.; Xing, R.; Liu, S. Self-cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Quan, Y.Y.; Zhang, L.Z. Experimental investigation of the anti-dust effect of transparent hydrophobic coatings applied for solar cell covering glass. Sol. Energy Mater. Sol. Cells 2017, 160, 382389. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, H. Self-cleaning performance of super-hydrophilic coatings for dust deposition reduction on solar photovoltaic cells. Coatings 2021, 11, 1059. [Google Scholar] [CrossRef]
- Meena, M.K.; Sinhamahapatra, A.; Aditya Kumar, A. Superhydrophobic polymer composite coating on glass via spin coating technique. Colloid Polym. Sci. 2019, 297, 1499–1505. [Google Scholar] [CrossRef]
- Mansour, A.; Mansour, H.; Al-Dawery, S.K. Sustainable self-cleaning treatments for architectural facades in developing countries. Alex. Eng. J. 2018, 57, 867–873. [Google Scholar] [CrossRef]
- Mosquera, S.K.M.J.; Addou, M.; Gil, M.L.A. Cu-TiO2/SiO2 photocatalysts for concrete-based building materials: Self-cleaning and air de-pollution performance. Constr. Build. Mater. 2021, 313, 125419. [Google Scholar] [CrossRef]
- Shen, L.; Hu, H.; Wang, S.; Fu, H. Preparation of super hydrophobic mMoS2/PDMS coating for fabrics. React. Funct. Polym. 2019, 143, 104315. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A review on self-cleaning coatings. J. Mater. Chem. 2011, 11, 16304. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B 2015, 176–177, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 32623291. [Google Scholar] [CrossRef] [PubMed]
- Krishna1, M.G.; Minjanampati, M.; Purkayastha, D.D. Metal oxide thin films and nanostructures for self-cleaning applications: Current status and future prospects. Eur. Phys. J. Appl. Phys. 2013, 62, 3001. [Google Scholar] [CrossRef] [Green Version]
- Saxena, N.; Naik, T.; Paria, S. Organization of SiO2 and TiO2 nanoparticles into fractal patterns on glass surface for the generation of superhydrophilicity. J. Phys. Chem. C 2017, 121, 2428–2436. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Kumar, K.S.S.; Mathew, D.; Nair, C.P.R. A robust, melting class bulk superhydrophobic material with heat-healing and self-cleaning properties. Sci. Rep. 2016, 5, 18510. [Google Scholar] [CrossRef] [Green Version]
- Kamegawa, T.; Irikawa, K.; Yamashita, H. Multifunctional surface designed by nanocomposite coating of polytetrafluoroethylene and TiO2 photocatalyst: Self-cleaning and superhydrophobicity. Sci. Rep. 2017, 7, 13628. [Google Scholar] [CrossRef] [Green Version]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804. [Google Scholar] [CrossRef]
- Ren, Y.; Li, W.; Cao, Z.; Jiao, Y.; Xu, J.; Liu, P.; Li, S.; Li, X. Robust TiO2 nanorods-SiO2 core-shell coating with high-performance self-cleaning properties under visible light. Appl. Surf. Sci. 2020, 509, 145377. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Cai, L. Facial synthesis of K0·3WO3/Ag nanocomposites for self-cleaning energy efficient window coatings. J. Alloys Compd. 2021, 856, 157069. [Google Scholar] [CrossRef]
- Nundy, S.; Ghosh, A.; Mallick, T.K. Hydrophilic and superhydrophilic self-cleaning coatings by morphologically varying ZnO microstructures for photovoltaic and glazing applications. ACS Omega 2020, 5, 1033–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamri, H.; Shahrani, A.A.; Bovero, E.; Khaldi, T.; Alabedi, G.; Obaid, W.; Al-Taie, I.; Fihri, A. Self-cleaning superhydrophobic epoxy coating based on fibrous silica-coated iron oxide magnetic nanoparticles. J. Colloid Interface Sci. 2018, 513, 349–356. [Google Scholar] [CrossRef]
- Euvananont, C.; Inpor, C.J.K.; Limthongkul, P.; Thanachayanont, C. TiO2 optical coating layers for self-cleaning applications. Ceram. Int. 2008, 34, 1067–1071. [Google Scholar] [CrossRef]
- Afzal, A.; Habib, A.; Ulhasan, I.; Shahid, M.; Rehman, A. Antireflective self-cleaning TiO2 coatings for solar energy harvesting applications. Front. Mater. 2021, 8, 687059. [Google Scholar] [CrossRef]
- Adachi, T.; Latthe, S.S.; Gosavi, S.W.; Roy, N.; Suzuki, N.; Ikari, H.; Kato, K.; Katsumata, K.; Nakata, K.; Furudate, M.; et al. Photocatalytic, superhydrophilic, self-cleaning TiO2 coating on cheap, light-weight, flexible polycarbonate substrates. Appl. Surf. Sci. 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Paolini, R.; Borroni, D.; Pedeferri, M.; Diamanti, M.V. Self-cleaning building materials: The multifaceted effects of titanium dioxide. Constr. Build. Mater. 2018, 182, 126133. [Google Scholar] [CrossRef] [Green Version]
- Calia, A.; Lettieri, M.; Masieri, M.; Pal, S.; Licciulli, A.; Arima, V. Limestones coated with photocatalytic TiO2 to enhance building surface with self-cleaning and depolluting abilities. J. Clean. Prod. 2017, 165, 10361047. [Google Scholar] [CrossRef]
- Kameya, Y.; Yabe, H. Optical and super hydrophilic characteristics of TiO2 coating with subwavelength surface structure consisting of spherical nanoparticle aggregates. Coatings 2019, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Chemin, J.B.; Bulou, S.; Baba, K.; Fontaine, C.; Sindzingre, T.; Boscher, N.D.; Choquet, P. Transparent anti-fogging and self-cleaning TiO2/SiO2 thin films on polymer substrates using atmospheric plasma. Sci. Rep. 2018, 8, 9603. [Google Scholar] [CrossRef]
- Rosales, A.; Ortiz-Frade, L.; Medina-Ramirez, I.E.; Godínez, L.A.; Esquivel, K. Self-cleaning of SiO2-TiO2 coating: Effect of sonochemical synthetic parameters on the morphological, mechanical, and photocatalytic properties of the films. Ultrason. Sonochem. 2021, 73, 105483. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.J.; Lin, K.T.; Kuo, Y.M.; Tsai, C.H. Preparation of high-transparency, superhydrophilic visible photo-induced photocatalytic film via a rapid plasma-modification process. Coatings 2021, 11, 784. [Google Scholar] [CrossRef]
- Kim, B.M.; Kim, J.K. Enhanced self-cleaning performance of Ag-F-codoped TiO2/SiO2 thin films. Korean J. Mater. Res. 2018, 28, 620–626. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, M.; Miao, X.; Zhang, L.; Wang, X. Improvement of the mechanical properties of nano-TiO2/poly(vinyl alcohol) composites by enhanced interaction between nanofiller and matrix. Polym. Compos. 2010, 31, 1184–1193. [Google Scholar] [CrossRef]
- Li, X.W.; Song, R.G.; Jiang, Y.; Wang, C.; Jiang, D. Surface modification of TiO2 nanoparticles and its effect on the properties of fluoropolymer/TiO2 nanocomposite coatings. Appl. Surf. Sci. 2013, 276, 761–768. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Nguyen, T.D.; Vu, D.T.; Dinh, D.P.; Nguyen, A.H.; Lien Ly, T.N.L.; Dao, P.H.; Nguyen, T.L.; Bach, L.G.; Thai, H. Modification of titanium dioxide nanoparticles with 3-(trimethoxysilyl) propyl methacrylate silane coupling agent. J. Chem. 2020, 2020, 1381407. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Zhang, C. TiO2-KH550 nanoparticle-reinforced PVA/xylan composite films with multifunctional properties. Materials 2018, 11, 1589. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Hu, Y.; Yan, S.; Shan, W.; Wei, C. Preparation of mesoporous SiO2/Bi2O3/TiO2 superhydrophilic thin films and their surface self-cleaning properties. RSC Adv. 2017, 7, 1966–1974. [Google Scholar] [CrossRef] [Green Version]
- Pakdel, E.; Daoud, W.A.; Wang, X. Self-cleaning and superhydrophilic wool by TiO2/SiO2 nanocomposite. Appl. Surf. Sci. 2013, 75, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Adak, D.; Ghosh, S.; Chakrabarty, P.; Mondala, A.; Saha, H.; Mukherjee, R.; Bhattacharyya, R. Self-cleaning V-TiO2:SiO2 thin-film coatings with enhanced transmission for solar glass cover and related applications. Sol. Energy 2017, 155, 410–418. [Google Scholar] [CrossRef]
- Sun, X.; Yi, M.; Feng, B.; Liu, R.; Sun, L.; Zhai, L.; Cao, H.; Zou, C. Shape-stabilized composite phase change material PEG@TiO2 through in situ encapsulation of PEG into 3D nanoporous TiO2 for thermal energy storage. Renew. Energy 2021, 170, 27–37. [Google Scholar] [CrossRef]
- Glu, E.K.; Deligoz, H.; Sozeri, H.; Baykal, A.; Toprak, M.S. Hydrothermal synthesis and characterization of PEG-Mn3O4 nanocomposite. Nano-Micro Lett. 2011, 3, 25–33. [Google Scholar] [CrossRef]
- Andrea, L.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-methoxyestradiol. Appl. Surf. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Ajra, H.; Marjina, L.; Mateja, P.; Aljosa, K.; Andreza, G.; Aleksandra, L. Novel reusable functionalized magnetic cobalt ferrite nanoparticles as oil adsorbents. Adsorpt. Sci. Technol. 2020, 38, 1–23. [Google Scholar] [CrossRef]
- Guermat, N.; Bellel, A.; Sahli, S.; Segui, Y.; Raynaud, P. Electrical and structural characterization of plasma-polymerized TEOS thin films as humidity sensors. Moroc. J. Condens. Matter 2010, 12, 208–211. [Google Scholar]
- Vronic, L.; Pierre, B.; Gabrielle, B. Metal oxide sol-gels (ZrO2, AlO (OH), and SiO2) to improve the mechanical Performance of Wood Substrates. J. Nanoparticles 2013, 2013, 273204. [Google Scholar] [CrossRef]
- Isoda, T.; Maeda, R. Development of an interaction assay between single-stranded nucleic acids trapped with silica particles and fluorescent compounds. J. Funct. Biomater. 2012, 3, 601. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Bai, H.; Huang, Y.; Liu, S.; Yen, S.; Tseng, Y. Compositing semiconductor photocatalysts and their microstructure modulation. Int. J. Photoenergy 2012, 2012, 620764. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.N.; Lee, S.-J.; Kim, C.-L. Fabrication of TiO2 ̶ KH550 ̶ PEG Super-Hydrophilic Coating on Glass Surface without UV/Plasma Treatment for Self-Cleaning and Anti-Fogging Applications. Materials 2022, 15, 3292. https://doi.org/10.3390/ma15093292
Hossain MN, Lee S-J, Kim C-L. Fabrication of TiO2 ̶ KH550 ̶ PEG Super-Hydrophilic Coating on Glass Surface without UV/Plasma Treatment for Self-Cleaning and Anti-Fogging Applications. Materials. 2022; 15(9):3292. https://doi.org/10.3390/ma15093292
Chicago/Turabian StyleHossain, Muhammad Nobi, Sung-Jun Lee, and Chang-Lae Kim. 2022. "Fabrication of TiO2 ̶ KH550 ̶ PEG Super-Hydrophilic Coating on Glass Surface without UV/Plasma Treatment for Self-Cleaning and Anti-Fogging Applications" Materials 15, no. 9: 3292. https://doi.org/10.3390/ma15093292
APA StyleHossain, M. N., Lee, S. -J., & Kim, C. -L. (2022). Fabrication of TiO2 ̶ KH550 ̶ PEG Super-Hydrophilic Coating on Glass Surface without UV/Plasma Treatment for Self-Cleaning and Anti-Fogging Applications. Materials, 15(9), 3292. https://doi.org/10.3390/ma15093292