Towards Room Temperature Phase Transition of W-Doped VO2 Thin Films Deposited by Pulsed Laser Deposition: Thermochromic, Surface, and Structural Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of W-Doped VO2 Thin Films Using Pulsed Laser Deposition
2.2. Characterization of the Undoped VO2 and W-Doped VO2 Films
3. Results and Discussion
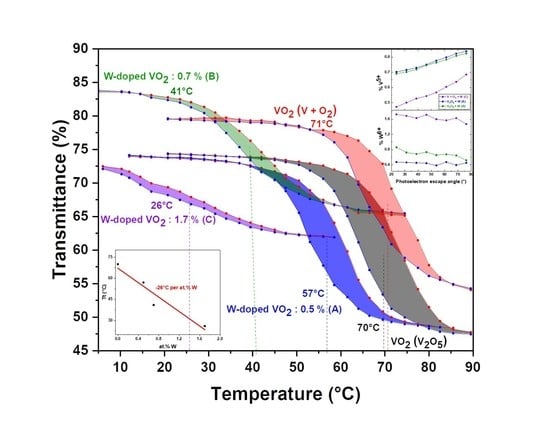
3.1. Thermochromic Properties
3.2. Surface Chemistry Analysis: Composition and Depth Distribution
3.3. Structural and Morphological Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Wang, Z.; Ren, H.; Chen, Y.; Yan, W.; Wang, C.; Li, B.; Jiang, J.; Zou, C. Gate-Controlled VO2 Phase Transition for High-Performance Smart Windows. Sci. Adv. 2019, 5, eaav6815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizsei, J.; Lappalainen, J.; Pohl, L. Active Thermal-Electronic Devices Based on Heat-Sensitive Metal-Insulator-Transition Resistor Elements. Sens. Actuators A Phys. 2017, 267, 14–20. [Google Scholar] [CrossRef]
- Beaumont, A.; Leroy, J.; Orlianges, J.-C.; Crunteanu, A. Current-Induced Electrical Self-Oscillations across out-of-Plane Threshold Switches Based on VO2 Layers Integrated in Crossbars Geometry. J. Appl. Phys. 2014, 115, 154502. [Google Scholar] [CrossRef]
- Vardi, N.; Anouchi, E.; Yamin, T.; Middey, S.; Kareev, M.; Chakhalian, J.; Dubi, Y.; Sharoni, A. Ramp-Reversal Memory and Phase-Boundary Scarring in Transition Metal Oxides. Adv. Mater. 2017, 29, 1605029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Lee, D.; Cho, S.J.; Seo, J.-H.; Liu, D.; Eom, C.-B.; Ma, Z. Epitaxial VO2 Thin Film-Based Radio-Frequency Switches with Thermal Activation. Appl. Phys. Lett. 2017, 111, 063110. [Google Scholar] [CrossRef]
- Ji, Y.D.; Pan, T.S.; Bi, Z.; Liang, W.Z.; Zhang, Y.; Zeng, H.Z.; Wen, Q.Y.; Zhang, H.W.; Chen, C.L.; Jia, Q.X.; et al. Epitaxial Growth and Metal-Insulator Transition of Vanadium Oxide Thin Films with Controllable Phases. Appl. Phys. Lett. 2012, 101, 071902. [Google Scholar] [CrossRef]
- Burkhardt, W.; Christmann, T.; Meyer, B.K.; Niessner, W.; Schalch, D.; Scharmann, A. W- and F-Doped VO2 Films Studied by Photoelectron Spectrometry. Thin Solid Film. 1999, 345, 229–235. [Google Scholar] [CrossRef]
- Guan, S.; Souquet-Basiège, M.; Toulemonde, O.; Denux, D.; Penin, N.; Gaudon, M.; Rougier, A. Toward Room-Temperature Thermochromism of VO2 by Nb Doping: Magnetic Investigations. Chem. Mater. 2019, 31, 9819–9830. [Google Scholar] [CrossRef]
- Khan, G.R.; Asokan, K.; Ahmad, B. Room Temperature Tunability of Mo-Doped VO2 Nanofilms across Semiconductor to Metal Phase Transition. Thin Solid Film. 2017, 625, 155–162. [Google Scholar] [CrossRef]
- Piccirillo, C.; Binions, R.; Parkin, I.P. Nb-Doped VO2 Thin Films Prepared by Aerosol-Assisted Chemical Vapour Deposition. Eur. J. Inorg. Chem. 2007, 2007, 4050–4055. [Google Scholar] [CrossRef]
- Chen, S.-E.; Lu, H.-H.; Brahma, S.; Huang, J.-L. Effects of Annealing on Thermochromic Properties of W-Doped Vanadium Dioxide Thin Films Deposited by Electron Beam Evaporation. Thin Solid Film. 2017, 644, 52–56. [Google Scholar] [CrossRef]
- Romanyuk, A.; Steiner, R.; Marot, L.; Oelhafen, P. Temperature-Induced Metal–Semiconductor Transition in W-Doped VO2 Films Studied by Photoelectron Spectroscopy. Sol. Energy Mater. Sol. Cells 2007, 91, 1831–1835. [Google Scholar] [CrossRef]
- Tang, C.; Georgopoulos, P.; Fine, M.E.; Cohen, J.B.; Nygren, M.; Knapp, G.S.; Aldred, A. Local Atomic and Electronic Arrangements in WxV1−xO2. Phys. Rev. B 1985, 31, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Tazawa, M.; Ikeyama, M.; Tanemura, S.; Macák, K.; Wang, X.; Olafsson, S.; Helmersson, U. Growth and Characterization of Epitaxial Films of Tungsten-Doped Vanadium Oxides on Sapphire (110) by Reactive Magnetron Sputtering. J. Vac. Sci. Technol. A 1999, 17, 1817–1821. [Google Scholar] [CrossRef]
- Lee, Y.W.; Kim, B.-J.; Lim, J.-W.; Yun, S.J.; Choi, S.; Chae, B.-G.; Kim, G.; Kim, H.-T. Metal-Insulator Transition-Induced Electrical Oscillation in Vanadium Dioxide Thin Film. Appl. Phys. Lett. 2008, 92, 162903. [Google Scholar] [CrossRef]
- Karaoglan-Bebek, G.; Hoque, M.N.F.; Holtz, M.; Fan, Z.; Bernussi, A.A. Continuous Tuning of W-Doped VO2 Optical Properties for Terahertz Analog Applications. Appl. Phys. Lett. 2014, 105, 201902. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Wang, S.; Lu, M.; Zuo, L. A Metal-Insulator Transition Study of VO2 Thin Films Grown on Sapphire Substrates. J. Appl. Phys. 2017, 122, 235102. [Google Scholar] [CrossRef]
- Sahana, M.B.; Subbanna, G.N.; Shivashankar, S.A. Phase Transformation and Semiconductor-Metal Transition in Thin Films of VO2 Deposited by Low-Pressure Metalorganic Chemical Vapor Deposition. J. Appl. Phys. 2002, 92, 6495–6504. [Google Scholar] [CrossRef]
- Ahmed, N.; Mahmood, R.; Umar, Z.A.; Liaqat, U.; ul Haq, M.A.; Ahmed, R.; Ahmad, P.; Raza, S.R.A.; Baig, M.A. Near Room Temperature, SMT and Visible Photo-Response in Pulsed Laser Deposited VO2 (M1) Thin Films. Phys. B Condens. Matter 2022, 635, 413841. [Google Scholar] [CrossRef]
- Umar, Z.A.; Ahmed, N.; Ahmed, R.; Arshad, M.; Anwar-Ul-Haq, M.; Hussain, T.; Baig, M.A. Substrate Temperature Effects on the Structural, Compositional, and Electrical Properties of VO2 Thin Films Deposited by Pulsed Laser Deposition. Surf. Interface Anal. 2018, 50, 297–303. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Goswami, A.; Umarji, A.M. Synthesis of Phase Pure Vanadium Dioxide (VO2) Thin Film by Reactive Pulsed Laser Deposition. J. Appl. Phys. 2018, 124, 135301. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Jamon, D.; Loir, A.-S.; Garrelie, F.; Donnet, C. Tailoring Thermochromic and Optical Properties of VO2 Thin Films by Pulsed Laser Deposition Using Different Starting Routes. Opt. Mater. 2022, 133, 113004. [Google Scholar] [CrossRef]
- Mulchandani, K.; Soni, A.; Pathy, K.; Mavani, K.R. Structural Transformation and Tuning of Electronic Transitions by W-Doping in VO2 Thin Films. Superlattices Microstruct. 2021, 154, 106883. [Google Scholar] [CrossRef]
- Émond, N.; Ibrahim, A.; Torriss, B.; Hendaoui, A.; Al-Naib, I.; Ozaki, T.; Chaker, M. Impact of Tungsten Doping on the Dynamics of the Photo-Induced Insulator-Metal Phase Transition in VO2 Thin Film Investigated by Optical Pump-Terahertz Probe Spectroscopy. Appl. Phys. Lett. 2017, 111, 092105. [Google Scholar] [CrossRef]
- Soltani, M.; Chaker, M.; Haddad, E.; Kruzelecky, R.V.; Nikanpour, D. Optical Switching of Vanadium Dioxide Thin Films Deposited by Reactive Pulsed Laser Deposition. J. Vac. Sci. Technol. A 2004, 22, 859–864. [Google Scholar] [CrossRef]
- Majid, S.S.; Sahu, S.R.; Ahad, A.; Dey, K.; Gautam, K.; Rahman, F.; Behera, P.; Deshpande, U.; Sathe, V.G.; Shukla, D.K. Role of V-V Dimerization in the Insulator-Metal Transition and Optical Transmittance of Pure and Doped VO2 Thin Films. Phys. Rev. B 2020, 101, 014108. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, J.B. The Two Components of the Crystallographic Transition in VO2. J. Solid State Chem. 1971, 3, 490–500. [Google Scholar] [CrossRef]
- Asayesh-Ardakani, H.; Nie, A.; Marley, P.M.; Zhu, Y.; Phillips, P.J.; Singh, S.; Mashayek, F.; Sambandamurthy, G.; Low, K.; Klie, R.F.; et al. Atomic Origins of Monoclinic-Tetragonal (Rutile) Phase Transition in Doped VO2 Nanowires. Nano Lett. 2015, 15, 7179–7188. [Google Scholar] [CrossRef]
- Tan, X.; Yao, T.; Long, R.; Sun, Z.; Feng, Y.; Cheng, H.; Yuan, X.; Zhang, W.; Liu, Q.; Wu, C.; et al. Unraveling Metal-Insulator Transition Mechanism of VO2Triggered by Tungsten Doping. Sci. Rep. 2012, 2, 466. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Sang, J.; Hua, Z.; Xu, L.; Xu, X.; Wang, C.; Wu, B. A Simple Method for Synthesizing VO2 with Almost Coincident Hysteresis Loops on Si Substrate Containing TiO2 Buffer Layer. J. Alloys Compd. 2021, 865, 158755. [Google Scholar] [CrossRef]
- Suh, J.Y.; Lopez, R.; Feldman, L.C.; Haglund, R.F. Semiconductor to Metal Phase Transition in the Nucleation and Growth of VO2 Nanoparticles and Thin Films. J. Appl. Phys. 2004, 96, 1209–1213. [Google Scholar] [CrossRef]
- Jin, P.; Nakao, S.; Tanemura, S. Tungsten Doping into Vanadium Dioxide Thermochromic Films by High-Energy Ion Implantation and Thermal Annealing. Thin Solid Film. 1998, 324, 151–158. [Google Scholar] [CrossRef]
- Lopez, R.; Haynes, T.E.; Boatner, L.A.; Feldman, L.C.; Haglund, R.F. Size Effects in the Structural Phase Transition of VO2 Nanoparticles. Phys. Rev. B 2002, 65, 224113. [Google Scholar] [CrossRef]
- Hu, L.; Tao, H.; Chen, G.; Pan, R.; Wan, M.; Xiong, D.; Zhao, X. Porous W-Doped VO2 Films with Simultaneously Enhanced Visible Transparency and Thermochromic Properties. J. Sol-Gel Sci. Technol. 2016, 77, 85–93. [Google Scholar] [CrossRef]
- Li, B.; Tian, S.; Tao, H.; Zhao, X. Tungsten Doped M-Phase VO2 Mesoporous Nanocrystals with Enhanced Comprehensive Thermochromic Properties for Smart Windows. Ceram. Int. 2019, 45, 4342–4350. [Google Scholar] [CrossRef]
- Shen, N.; Chen, S.; Shi, R.; Niu, S.; Amini, A.; Cheng, C. Phase Transition Hysteresis of Tungsten Doped VO2 Synergistically Boosts the Function of Smart Windows in Ambient Conditions. ACS Appl. Electron. Mater. 2021, 3, 3648–3656. [Google Scholar] [CrossRef]
- Zomaya, D.; Xu, W.Z.; Grohe, B.; Mittler, S.; Charpentier, P.A. W-Doped VO2/PVP Coatings with Enhanced Thermochromic Performance. Sol. Energy Mater. Sol. Cells 2019, 200, 109900. [Google Scholar] [CrossRef]
- Zou, J.; Chen, X.; Xiao, L. Phase Transition Performance Recovery of W-Doped VO2 by Annealing Treatment. Mater. Res. Express 2018, 5, 065055. [Google Scholar] [CrossRef]
- Dang, Y.; Zhao, L.; Liu, J. Preparation and Optical Properties of W-Doped VO2/AZO Bilayer Composite Film. Ceram. Int. 2020, 46, 9079–9085. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, F.; Yao, J.; Zhu, T.; Xia, H.; Yang, G.; Liu, B.; Gao, Y. Facile Synthesis, Formation Mechanism and Thermochromic Properties of W-Doped VO2 (M) Nanoparticles for Smart Window Applications. J. Mater. Chem. C 2020, 8, 13396–13404. [Google Scholar] [CrossRef]
- Shen, N.; Chen, S.; Chen, Z.; Liu, X.; Cao, C.; Dong, B.; Luo, H.; Liu, J.; Gao, Y. The Synthesis and Performance of Zr-Doped and W–Zr-Codoped VO2 Nanoparticles and Derived Flexible Foils. J. Mater. Chem. A 2014, 2, 15087–15093. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, L.; Meng, W.; Zhong, C.; Wei, S.; Dong, B.; Xu, Z.; Wan, L.; Wang, S. Tungsten-Doped Vanadium Dioxide Thin Films as Smart Windows with Self-Cleaning and Energy-Saving Functions. J. Alloys Compd. 2017, 694, 124–131. [Google Scholar] [CrossRef]
- Wang, S.; Wei, W.; Huang, T.; Yuan, M.; Yang, Y.; Yang, W.; Zhang, R.; Zhang, T.; Chen, Z.; Chen, X.; et al. Al-Doping-Induced VO2 (B) Phase in VO2 (M) Toward Smart Optical Thin Films with Modulated ΔTvis and ΔTc. Adv. Eng. Mater. 2019, 21, 1900947. [Google Scholar] [CrossRef]
- Takami, H.; Kanki, T.; Ueda, S.; Kobayashi, K.; Tanaka, H. Electronic Structure of W-Doped VO2 Thin Films with Giant Metal–Insulator Transition Investigated by Hard X-Ray Core-Level Photoemission Spectroscopy. Appl. Phys. Express 2010, 3, 063201. [Google Scholar] [CrossRef]
- Sawatzky, G.A.; Post, D. X-Ray Photoelectron and Auger Spectroscopy Study of Some Vanadium Oxides. Phys. Rev. B 1979, 20, 1546–1555. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Chen, Z.; Du, J.; Cao, C.; Kang, L.; Luo, H. Thermochromic VO2 Thin Films: Solution-Based Processing, Improved Optical Properties, and Lowered Phase Transformation Temperature. Langmuir 2010, 26, 10738–10744. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Z.; Wang, C.; Deng, S.; Yang, J.; Wang, X.; Li, J.; Zhao, Y.; Jin, H. Influence of the Charge Compensation Effect on the Metal–Insulator Transition of Mg-W Co-Doped VO2. Appl. Surf. Sci. 2022, 579, 151990. [Google Scholar] [CrossRef]
- Ström, P.; Primetzhofer, D. In-Situ Measurement of Diffusion and Surface Segregation of W and Ta in Bare and W-Coated EUROFER97 during Thermal Annealing. Nucl. Mater. Energy 2021, 27, 100979. [Google Scholar] [CrossRef]
- Demeter, M.; Neumann, M.; Reichelt, W. Mixed-Valence Vanadium Oxides Studied by XPS. Surf. Sci. 2000, 454–456, 41–44. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS Binding Energies for Different Vanadium Oxidation States (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.; Kim, J.W.; Lee, I.J.; Kim, Y.; Yun, H.-J.; Lee, J.; Park, S. Hydrogen Incorporation Induced the Octahedral Symmetry Variation in VO2 Films. Appl. Surf. Sci. 2017, 396, 36–40. [Google Scholar] [CrossRef]
- Shvets, P.; Dikaya, O.; Maksimova, K.; Goikhman, A. A Review of Raman Spectroscopy of Vanadium Oxides. J. Raman Spectrosc. 2019, 50, 1226–1244. [Google Scholar] [CrossRef]
- Schilbe, P. Raman Scattering in VO2. Phys. B Condens. Matter 2002, 316–317, 600–602. [Google Scholar] [CrossRef]
- Srivastava, R.; Chase, L.L. Raman Spectrum of Semiconducting and Metallic VO2. Phys. Rev. Lett. 1971, 27, 727–730. [Google Scholar] [CrossRef]
- Gu, D.; Zhou, X.; Sun, Z.; Jiang, Y. Influence of Gadolinium-Doping on the Microstructures and Phase Transition Characteristics of VO2 Thin Films. J. Alloys Compd. 2017, 705, 64–69. [Google Scholar] [CrossRef]
- Ji, C.; Wu, Z.; Wu, X.; Feng, H.; Wang, J.; Huang, Z.; Zhou, H.; Yao, W.; Gou, J.; Jiang, Y. Optimization of Metal-to-Insulator Phase Transition Properties in Polycrystalline VO2 Films for Terahertz Modulation Applications by Doping. J. Mater. Chem. C 2018, 6, 1722–1730. [Google Scholar] [CrossRef]
- Whittaker, L.; Wu, T.-L.; Stabile, A.; Sambandamurthy, G.; Banerjee, S. Single-Nanowire Raman Microprobe Studies of Doping-, Temperature-, and Voltage-Induced Metal–Insulator Transitions of WxV1−XO2 Nanowires. ACS Nano 2011, 5, 8861–8867. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.-W.; Chen, F.; Yu, L.; Chen, X. Tuning Phase Transition Temperature of VO2 Thin Films by Annealing Atmosphere. J. Phys. D Appl. Phys. 2015, 48, 265104. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Ling, C.; Zhao, X.; Zhao, Y.; Jin, H. Electric Field Driven Abnormal Increase in Conductivity of Tungsten-Doped VO2 Nanofilms. Thin Solid Film. 2021, 725, 138643. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Tatarenko, A.Y.; Gorodetsky, A.A.; Makarevich, O.N.; Navarro-Cía, M.; Makarevich, A.M.; Kaul, A.R.; Eliseev, A.A.; Boytsova, O.V. Fabrication of Epitaxial W-Doped VO2 Nanostructured Films for Terahertz Modulation Using the Solvothermal Process. ACS Appl. Nano Mater. 2021, 4, 10592–10600. [Google Scholar] [CrossRef]
- Pan, G.; Yin, J.; Ji, K.; Li, X.; Cheng, X.; Jin, H.; Liu, J. Synthesis and Thermochromic Property Studies on W Doped VO2 Films Fabricated by Sol-Gel Method. Sci. Rep. 2017, 7, 6132. [Google Scholar] [CrossRef] [PubMed]








 | Sample A | Sample B | Sample C |
|---|---|---|---|
| V ablated thickness per layer (nm) | / | / | 0.283 |
| V2O5 ablated thickness per layer (nm) | 0.283 | 0.270 | / |
| W ablated thickness per layer (nm) | 0.015 | 0.03 | 0.015 |
| Number of VOx and W layers | 67 | 67 | 67 |
| Total thickness (nm) | 20 | 20 | 20 |
| W (at. %) | Tt (°C) | ∆T (°C) | Tlum | ∆Tsol | Process | Structural Form | Ref. |
|---|---|---|---|---|---|---|---|
| 0.14 | 64 | 6.8 | Unchanged | Decreased | Electron beam evaporation | Thin film | [11] |
| 2 | 28 | 15 | Unchanged | Decreased | Spin coating | Thin film | [37] |
| 0.4 | 43 | / | Increased | Decreased | Hydrothermal | Mesoporous film | [38] |
| 0.8 | 36 | 20.5 | Decreased | Decreased | Hydrothermal | Nanoparticles | [39] |
| 3 | 44.4 | 18.4 | Increased | Increased | Spin coating | Coating | [40] |
| 2 | 43 | / | Decreased | Decreased | Microemulsion technology | Nanostructure | [41] |
| 1.3 | 55 | / | Decreased | Decreased | Magnetron sputtering | Thin film | [42] |
| 1.3 | 17.5 | 25.1 | Increased | Decreased | Hydrothermal | Nanoparticle | [43] |
| 0.7 | 41 | 6 | Increased | Decreased | PLD | Thin film | This work |
| 1.7 | 26 | 4 | Increased | Decreased | PLD | Thin film | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleu, Y.; Bourquard, F.; Barnier, V.; Loir, A.-S.; Garrelie, F.; Donnet, C. Towards Room Temperature Phase Transition of W-Doped VO2 Thin Films Deposited by Pulsed Laser Deposition: Thermochromic, Surface, and Structural Analysis. Materials 2023, 16, 461. https://doi.org/10.3390/ma16010461
Bleu Y, Bourquard F, Barnier V, Loir A-S, Garrelie F, Donnet C. Towards Room Temperature Phase Transition of W-Doped VO2 Thin Films Deposited by Pulsed Laser Deposition: Thermochromic, Surface, and Structural Analysis. Materials. 2023; 16(1):461. https://doi.org/10.3390/ma16010461
Chicago/Turabian StyleBleu, Yannick, Florent Bourquard, Vincent Barnier, Anne-Sophie Loir, Florence Garrelie, and Christophe Donnet. 2023. "Towards Room Temperature Phase Transition of W-Doped VO2 Thin Films Deposited by Pulsed Laser Deposition: Thermochromic, Surface, and Structural Analysis" Materials 16, no. 1: 461. https://doi.org/10.3390/ma16010461
APA StyleBleu, Y., Bourquard, F., Barnier, V., Loir, A. -S., Garrelie, F., & Donnet, C. (2023). Towards Room Temperature Phase Transition of W-Doped VO2 Thin Films Deposited by Pulsed Laser Deposition: Thermochromic, Surface, and Structural Analysis. Materials, 16(1), 461. https://doi.org/10.3390/ma16010461