Palladium-Phosphide-Modified Three-Dimensional Phospho-Doped Graphene Materials for Hydrogen Storage
Abstract
:1. Introduction
2. Material Preparation
2.1. Preparation of GO Solution
2.2. Preparation of 3D-Configuration Pd3P0.95/P-rGO at Different Thermal Reduction Temperatures
2.3. Computational Methodology
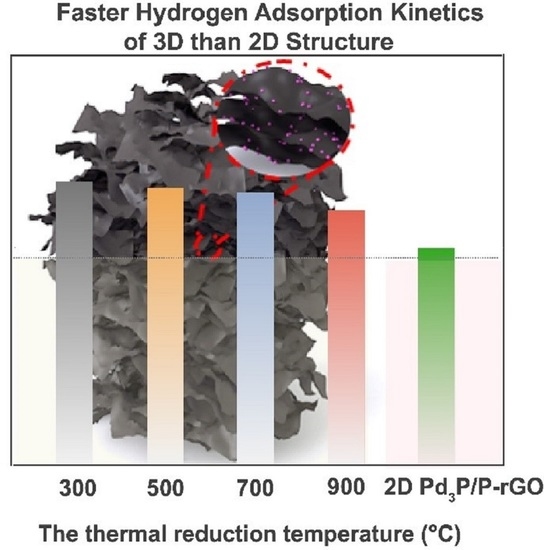
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.-M.; Li, G.-X.; Li, L. Comparative investigation on combustion characteristics of ADN-based liquid propellants in inert gas and oxidizing gas atmospheres with resistive ignition method. Fuel 2023, 334, 126742. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Z.; Bian, H.; Ding, M.; Meng, X. Identification and classification of the flow pattern of hydrogen-air-steam mixture gas under steam condensation. Int. J. Therm. Sci. 2023, 183, 107854. [Google Scholar] [CrossRef]
- Gao, X.; Li, B.; Sun, X.; Wu, B.; Hu, Y.; Ning, Z.; Li, J.; Wang, N. Engineering heterostructure and crystallinity of Ru/RuS2 nanoparticle composited with N-doped graphene as electrocatalysts for alkaline hydrogen evolution. Chin. Chem. Lett. 2021, 32, 3591–3595. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.M.; Wang, N.; Xu, Q.; Yu, J.H. Nanopore-Supported Metal Nanocatalysts for Efficient Hydrogen Generation from Liquid-Phase Chemical Hydrogen Storage Materials. Adv. Mater. 2020, 32, 2001818. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Li, X.; Kong, Q.; An, X.; Zhang, J.; Wang, Q.; Yao, W. Enhanced cycling stability and storage performance of Na0.67Ni0.33Mn0.67-xTixO1.9F0.1 cathode materials by Mn-rich shells and Ti doping. J. Colloid Interface Sci. 2023, 633, 82–91. [Google Scholar] [CrossRef]
- Yang, F.S.; Wang, J.; Zhang, Y.; Wu, Z.; Zhang, Z.X.; Zhao, F.Q.; Huot, J.; Novakovic, J.G.; Novakovic, N. Recent progress on the development of high entropy alloys (HEAs) for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2022, 47, 11236–11249. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Tao, H.; Fan, Q.; Ma, T.; Liu, S.; Gysling, H.; Texter, J.; Guo, F.; Sun, Z. Two-dimensional materials for energy conversion and storage. Prog. Mater. Sci. 2020, 111, 100637. [Google Scholar] [CrossRef]
- Qin, X.; Ola, O.; Zhao, J.; Yang, Z.; Tiwari, S.K.; Wang, N.; Zhu, Y. Recent Progress in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 1806. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Liu, C.; Fan, Y.Y.; Liu, M.; Cong, H.T.; Cheng, H.M.; Dresselhaus, M.S. Hydrogen storage in single-walled carbon nanotubes at room temperature. Science 1999, 286, 1127–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.; Tang, Q.; Zheng, Y.; Kidkhunthod, P.; Zhou, X.; Ji, B.; Tang, Y. High-entropy single-atom activated carbon catalysts for sustainable oxygen electrocatalysis. Nat. Sustain. 2023. [Google Scholar] [CrossRef]
- Chen, L.; Liang, X.; Wang, H.; Xiao, Q.; Qiu, X. Ultra-thin carbon nitride nanosheets for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 442, 136115. [Google Scholar] [CrossRef]
- Li, Z.; Li, K.; Du, P.; Mehmandoust, M.; Karimi, F.; Erk, N. Carbon-based photocatalysts for hydrogen production: A review. Chemosphere 2022, 308, 135998. [Google Scholar] [CrossRef]
- Yildirim, T.; Ciraci, S. Titanium-decorated carbon nanotubes as a potential high-capacity hydrogen storage medium. Phys. Rev. Lett. 2005, 94, 175501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, A.F.; Vajo, J.J.; Van Atta, S.L.; Olson, G.L. Enhanced hydrogen storage kinetics of LiBH4 in nanoporous carbon scaffolds. J. Phys. Chem. C 2008, 112, 5651–5657. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Z.; Han, Z.; Chu, D.; Chen, C.; Xie, X.; Shang, L.; Zhang, T. Highly dispersed platinum deposited on nitrogen-doped vertical graphene array for efficient electrochemical hydrogen evolution. 2D Mater. 2022, 9, 045011. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward Design of Synergistically Active Carbon-Based Catalysts for Electrocatalytic Hydrogen Evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef] [Green Version]
- Xiong, M.; Gao, Z.; Qin, Y. Spillover in Heterogeneous Catalysis: New Insights and Opportunities. ACS Catal. 2021, 11, 3159–3172. [Google Scholar] [CrossRef]
- Putri, L.K.; Ong, W.-J.; Chang, W.S.; Chai, S.-P. Heteroatom doped graphene in photocatalysis: A review. Appl. Surf. Sci. 2015, 358, 2–14. [Google Scholar] [CrossRef]
- Dobrota, A.S.; Pasti, I.A.; Mentus, S.V.; Johansson, B.; Skorodumova, N.V. Altering the reactivity of pristine, N- and P-doped graphene by strain engineering: A DFT view on energy-related aspects. Appl. Surf. Sci. 2020, 514, 145937. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Li, J.; Zhang, K.; Habibullah; Xia, G.; Wu, C.; Wang, Y.; Cen, W.; Chen, Y.; Yan, Y.; et al. Pd3P nanoparticles decorated P-doped graphene for high hydrogen storage capacity and stable hydrogen adsorption-desorption performance. Nano Energy 2022, 99, 107360. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.; Chen, J. Three-dimensional graphene-based composites for energy applications. Nanoscale 2015, 7, 6924–6943. [Google Scholar] [CrossRef] [Green Version]
- Kuang, P.; Zhu, B.; Li, Y.; Liu, H.; Yu, J.; Fan, K. Graphdiyne: A superior carbon additive to boost the activity of water oxidation catalysts. Nanoscale Horiz. 2018, 3, 317–326. [Google Scholar] [CrossRef]
- Jiang, H.; Lee, P.S.; Li, C. 3D carbon-based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- Bi, L.; Yin, J.; Huang, X.; Wang, Y.H.; Yang, Z.H. Graphene pillared with hybrid fullerene and nanotube as a novel 3D framework for hydrogen storage: A DFT and GCMC study. Int. J. Hydrogen Energy 2020, 45, 17637–17648. [Google Scholar] [CrossRef]
- Tylianakis, E.; Dimitrakakis, G.K.; Melchor, S.; Dobado, J.A.; Froudakis, G.E. Porous nanotube network: A novel 3-D nanostructured material with enhanced hydrogen storage capacity. Chem. Commun. 2011, 47, 2303–2305. [Google Scholar] [CrossRef]
- Lei, Y.P.; Shi, Q.; Han, C.; Wang, B.; Wu, N.; Wang, H.; Wang, Y.D. N-doped graphene grown on silk cocoon-derived interconnected carbon fibers for oxygen reduction reaction and photocatalytic hydrogen production. Nano Res. 2016, 9, 2498–2509. [Google Scholar] [CrossRef]
- Yan, D.F.; Li, F.; Xu, Y.Z.; Liu, C.B.; Wang, Y.; Tang, Y.H.; Yang, L.M.; Wang, L.L. Three-dimensional reduced graphene oxide-Mn3O4 nanosheet hybrid decorated with palladium nanoparticles for highly efficient hydrogen evolution. Int. J. Hydrogen Energy 2018, 43, 3369–3377. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, X.L.; Zhang, H.; Tang, Y.J.; Wang, J. Molecular dynamic investigations of hydrogen storage efficiency of graphene sheets with the bubble structure. Struct. Chem. 2015, 26, 531–537. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, F.; Song, H. Effect of reduction temperature on the structure and hydrodesulfurization performance of Na doped Ni2P/MCM-41 catalysts. RSC Adv. 2019, 9, 15488–15494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohtar, S.S.; Aziz, F.; Ismail, A.F.; Sambudi, N.S.; Abdullah, H.; Rosli, A.N.; Ohtani, B. Impact of Doping and Additive Applications on Photocatalyst Textural Properties in Removing Organic Pollutants: A Review. Catalysts 2021, 11, 1160. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, B.; Yuan, R. Doping a metal (Ag, Al, Mn, Ni and Zn) on TiO2 nanotubes and its effect on Rhodamine B photocatalytic oxidation. Environ. Eng. Res. 2015, 20, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, C.; Gupta, A.K. Characterization and photocatalytic performance evaluation of various metal ion-doped microstructured TiO2 under UV and visible light. J. Environ. Sci. Health Part A 2015, 50, 659–668. [Google Scholar] [CrossRef]
- Tan, T.Q.; Geng, L.; Yao, C.L.; Lin, Y.; He, Y. Study on the Effect of Reduction Temperature on the Catalytic Activity of Fe-Mo/Al2O3 Catalyst and the Microstructure of Carbon Nanotubes. Mater. Sci. Forum 2021, 1036, 114–121. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Zhang, Y.; Jiang, W.-J.; Zhang, X.; Dai, Z.; Wan, L.-J.; Hu, J.-S. Pomegranate-like N, P-Doped Mo2C@C Nanospheres as Highly Active Electrocatalysts for Alkaline Hydrogen Evolution. ACS Nano 2016, 10, 8851–8860. [Google Scholar] [CrossRef]
- Wang, T.-J.; Jiang, Y.-C.; He, J.-W.; Li, F.-M.; Ding, Y.; Chen, P.; Chen, Y. Porous palladium phosphide nanotubes for formic acid electrooxidation. Carbon Energy 2022, 4, 283–293. [Google Scholar] [CrossRef]
- Qiu, B.; Li, Q.; Shen, B.; Xing, M.; Zhang, J. Stober-like method to synthesize ultradispersed Fe3O4 nanoparticles on graphene with excellent Photo-Fenton reaction and high-performance lithium storage. Appl. Catal. B Environ. 2016, 183, 216–223. [Google Scholar] [CrossRef]
- Spyrou, K.; Gournis, D.; Rudolf, P. Hydrogen Storage in Graphene-Based Materials: Efforts Towards Enhanced Hydrogen Absorption. ECS J. Solid State Sci. Technol. 2013, 2, M3160–M3169. [Google Scholar] [CrossRef] [Green Version]
- Cabria, I.; López, M.J.; Alonso, J.A. Theoretical study of the transition from planar to three-dimensional structures of palladium clusters supported on graphene. Phys. Rev. B 2010, 81, 035403. [Google Scholar] [CrossRef]
- López, M.J.; Cabria, I.; Alonso, J.A. Palladium Clusters Anchored on Graphene Vacancies and Their Effect on the Reversible Adsorption of Hydrogen. J. Phys. Chem. C 2014, 118, 5081–5090. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.; Yang, S.; Wang, E.; Zhang, Z. Charged Fullerenes as High-Capacity Hydrogen Storage Media. Nano Lett. 2007, 7, 2578–2583. [Google Scholar] [CrossRef] [PubMed]
- Kubas, J. Dihydrogen Complexes as Prototypes for the Coordination Chemistry of Saturated Molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 6901–6907. [Google Scholar] [CrossRef] [Green Version]
- Henkelman, G.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, K.; Guan, C.; He, Z.; Lu, Z.; Chen, T.; Liu, J.; Tan, X.; Yang Tan, T.T.; Li, C.M. Surface functionalization-enhanced spillover effect on hydrogen storage of Ni–B nanoalloy-doped activated carbon. Int. J. Hydrogen Energy 2011, 36, 13663–13668. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chung, T.-Y.; Shen, C.-C.; Yu, M.-S.; Tsao, C.-S.; Shi, G.-N.; Huang, C.-C.; Ger, M.-D.; Lee, W.-L. Hydrogen storage performance in palladium-doped graphene/carbon composites. Int. J. Hydrogen Energy 2013, 38, 3681–3688. [Google Scholar] [CrossRef]
- Li, J.; Jin, C.; Qian, R.; Wu, C.; Wang, Y.; Yan, Y.; Chen, Y. Hydrogen absorption-desorption cycle decay mechanism of palladium nanoparticle decorated nitrogen-doped graphene. Prog. Nat. Sci. Mater. Int. 2021, 31, 514–520. [Google Scholar] [CrossRef]
- Wei, L.; Mao, Y. Enhanced hydrogen storage performance of reduced graphene oxide hybrids with nickel or its metallic mixtures based on spillover mechanism. Int. J. Hydrogen Energy 2016, 41, 11692–11699. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Z.; Wang, T. Enhanced hydrogen storage performance of three-dimensional hierarchical porous graphene with nickel nanoparticles. Int. J. Hydrogen Energy 2018, 43, 11120–11131. [Google Scholar] [CrossRef]






| Materials | Temperature (K) | Pressure a | H2 Storage (wt%) | Ref. |
|---|---|---|---|---|
| 3D graphene | 77 | 1 bar | 1.4 | [47] |
| Pd-graphene/carbon | 298 | 8 MPa | 0.82 | [48] |
| Pd/N-rGO | 298 | 4 MPa | 2.90 | [49] |
| Ni/Pd-rGO | 293.15 | 800 mmHg | 0.13 | [50] |
| 3DHPG-Ni-7.5 nanocomposite | 77 | 5 bar | 4.22 | [51] |
| 3DHPG-Ni-7.5 nanocomposite | 298 | 5 bar | 1.95 | [51] |
| 3D Pd3P0.95/P-rGO-300 | 298 | 4 MPa | 2.43 | This work |
| 3D Pd3P0.95/P-rGO-500 | 298 | 4 MPa | 3.79 | This work |
| 3D Pd3P0.95/P-rGO-700 | 298 | 4 MPa | 3.31 | This work |
| 3D Pd3P0.95/P-rGO-900 | 298 | 4 MPa | 3.05 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Habibullah; Xia, G.; Jin, C.; Wang, Y.; Yan, Y.; Chen, Y.; Gong, X.; Lai, Y.; Wu, C. Palladium-Phosphide-Modified Three-Dimensional Phospho-Doped Graphene Materials for Hydrogen Storage. Materials 2023, 16, 4219. https://doi.org/10.3390/ma16124219
Chen Y, Habibullah, Xia G, Jin C, Wang Y, Yan Y, Chen Y, Gong X, Lai Y, Wu C. Palladium-Phosphide-Modified Three-Dimensional Phospho-Doped Graphene Materials for Hydrogen Storage. Materials. 2023; 16(12):4219. https://doi.org/10.3390/ma16124219
Chicago/Turabian StyleChen, Yiwen, Habibullah, Guanghui Xia, Chaonan Jin, Yao Wang, Yigang Yan, Yungui Chen, Xiufang Gong, Yuqiu Lai, and Chaoling Wu. 2023. "Palladium-Phosphide-Modified Three-Dimensional Phospho-Doped Graphene Materials for Hydrogen Storage" Materials 16, no. 12: 4219. https://doi.org/10.3390/ma16124219
APA StyleChen, Y., Habibullah, Xia, G., Jin, C., Wang, Y., Yan, Y., Chen, Y., Gong, X., Lai, Y., & Wu, C. (2023). Palladium-Phosphide-Modified Three-Dimensional Phospho-Doped Graphene Materials for Hydrogen Storage. Materials, 16(12), 4219. https://doi.org/10.3390/ma16124219