Overview of Approaches to Increase the Electrochemical Activity of Conventional Perovskite Air Electrodes
Abstract
:1. Introduction
2. Key Functional Properties of LSM and LSCF Electrode Materials: Advantages and Drawbacks
3. Conventional and Advanced Techniques to Fabricate Electrode Layers
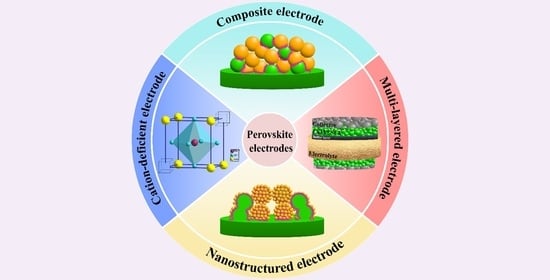
4. Methods to Improve the Electrochemical Performance of the Conventional Electrodes
4.1. Optimization of the Oxide Composition
4.2. Enhancement of the Ionic-Conducting Electrode Component
4.3. Improvement of the Electrode Surface
4.4. Improvement of the Electrode–Electrolyte Interface
5. Modeling of the Electrode Performance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erixno, O.; Rahim, N.A.; Ramadhani, F.; Adzman, N.N. Energy Management of Renewable Energy-Based Combined Heat and Power Systems: A Review. Sustain. Energy Technol. Assess. 2022, 51, 101944. [Google Scholar] [CrossRef]
- Iliev, I.K.; Filimonova, A.A.; Chichirov, A.A.; Chichirova, N.D.; Pechenkin, A.V.; Vinogradov, A.S. Theoretical and Experimental Studies of Combined Heat and Power Systems with SOFCs. Energies 2023, 16, 1898. [Google Scholar] [CrossRef]
- Lee, J.; Lin, K.-Y.A.; Jung, S.; Kwon, E.E. Hybrid Renewable Energy Systems Involving Thermochemical Conversion Process for Waste-to-Energy Strategy. Chem. Eng. J. 2023, 452, 139218. [Google Scholar] [CrossRef]
- He, V.; Gaffuri, M.; Van Herle, J.; Schiffmann, J. Readiness Evaluation of SOFC-MGT Hybrid Systems with Carbon Capture for Distributed Combined Heat and Power. Energy Convers. Manag. 2023, 278, 116728. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Q.; Liu, L.; Wu, Y.; Liu, H.; Gu, Z.; Zhu, C. A Review of Low and Zero Carbon Fuel Technologies: Achieving Ship Carbon Reduction Targets. Sustain. Energy Technol. Assess. 2022, 54, 102762. [Google Scholar] [CrossRef]
- Maestre, V.M.; Ortiz, A.; Ortiz, I. Challenges and Prospects of Renewable Hydrogen-Based Strategies for Full Decarbonization of Stationary Power Applications. Renew. Sustain. Energy Rev. 2021, 152, 111628. [Google Scholar] [CrossRef]
- Russo, M.A.; Carvalho, D.; Martins, N.; Monteiro, A. Forecasting the Inevitable: A Review on the Impacts of Climate Change on Renewable Energy Resources. Sustain. Energy Technol. Assess. 2022, 52, 102283. [Google Scholar] [CrossRef]
- Shen, M. Solid Oxide Fuel Cell-Lithium Battery Hybrid Power Generation System Energy Management: A Review. Int. J. Hydrogen Energy 2021, 46, 32974–32994. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, O. A Review of Solid Oxide Fuel Cell Based Hybrid Cycles. Int. J. Energy Res. 2022, 46, 8560–8589. [Google Scholar] [CrossRef]
- Feng, Y.; Qu, J.; Zhu, Y.; Wu, B.; Wu, Y.; Xiao, Z.; Liu, J. Progress and Prospect of the Novel Integrated SOFC-ICE Hybrid Power System: System Design, Mass and Heat Integration, System Optimization and Techno-Economic Analysis. Energy Convers. Manag. 2023, 18, 100350. [Google Scholar] [CrossRef]
- Kasaeian, A.; Javidmehr, M.; Mirzaie, M.R.; Fereidooni, L. Integration of Solid Oxide Fuel Cells with Solar Energy Systems: A Review. Appl. Therm. Eng. 2023, 224, 120117. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid Oxide Fuel Cell: Decade of Progress, Future Perspectives and Challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Bilal Hanif, M.; Motola, M.; Qayyum, S.; Rauf, S.; Khalid, A.; Li, C.-J.; Li, C.-X. Recent Advancements, Doping Strategies and the Future Perspective of Perovskite-Based Solid Oxide Fuel Cells for Energy Conversion. Chem. Eng. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Hu, S.; Li, J.; Zeng, Y.; Pu, J.; Chi, B. A Mini Review of the Recent Progress of Electrode Materials for Low-Temperature Solid Oxide Fuel Cells. Phys. Chem. Chem. Phys. 2023, 25, 5926–5941. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Filonova, E.A.; Ricote, S.; Medvedev, D.A.; Shao, Z. Chemical Design of Oxygen Electrodes for Solid Oxide Electrochemical Cells: A Guide. Sustain. Energy Technol. Assess. 2023, 57, 103185. [Google Scholar] [CrossRef]
- Md Harashid, M.A.; Chen, R.S.; Ahmad, S.H.; Ismail, A.F.; Baharuddin, N.A. Recent Advances in Electrode Material for Symmetrical Solid Oxide Fuel Cells and Way Forward Sustainability Based on Local Mineral Resources. Int. J. Energy Res. 2022, 46, 22188–22221. [Google Scholar] [CrossRef]
- Tahir, N.N.M.; Baharuddin, N.A.; Samat, A.A.; Osman, N.; Somalu, M.R. A Review on Cathode Materials for Conventional and Proton-Conducting Solid Oxide Fuel Cells. J. Alloys Compd. 2022, 894, 162458. [Google Scholar] [CrossRef]
- Zhang, M.; Du, Z.; Zhang, Y.; Zhao, H. Progress of Perovskites as Electrodes for Symmetrical Solid Oxide Fuel Cells. ACS Appl. Energy Mater. 2022, 5, 13081–13095. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G.; Pikalova, N.S.; Filonova, E.A. High-Entropy Materials in SOFC Technology: Theoretical Foundations for Their Creation, Features of Synthesis, and Recent Achievements. Materials 2022, 15, 8783. [Google Scholar] [CrossRef]
- Skutina, L.; Filonova, E.; Medvedev, D.; Maignan, A. Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells. Materials 2021, 14, 1715. [Google Scholar] [CrossRef]
- Curi, M.; da Silva, E.R.; de Furtado, J.G.M.; Ferraz, H.C.; Secchi, A.R. Anodes for SOFC: Review of Material Selection, Interface and Electrochemical Phenomena. Quim. Nova 2021, 44, 86–97. [Google Scholar] [CrossRef]
- Filonova, E.; Medvedev, D. Recent Progress in the Design, Characterisation and Application of LaAlO3− and LaGaO3-Based Solid Oxide Fuel Cell Electrolytes. Nanomaterials 2022, 12, 1991. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, I.; Kim, K.J.; Bae, K.T.; Kim, D.; Koo, J.; Yu, H.; Lee, K.T. A Brief Review of Heterostructure Electrolytes for High-Performance Solid Oxide Fuel Cells at Reduced Temperatures. J. Korean Ceram. Soc. 2022, 59, 131–152. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; Motola, M.; Babar, Z.U.D.; Li, C.-J.; Li, C.-X. Recent Progress of Perovskite-Based Electrolyte Materials for Solid Oxide Fuel Cells and Performance Optimizing Strategies for Energy Storage Applications. Mater. Res. Bull. 2022, 146, 111612. [Google Scholar] [CrossRef]
- Dey, S.; Chaudhary, S.; Parvatalu, D.; Mukhopadhyay, M.; Sharma, A.D.; Mukhopadhyay, J. Advancing Electrode Properties through Functionalization for Solid Oxide Cells Application: A Review. Chem. Asian J. 2023, 18, e202201222. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Review of Perovskite-Structure Related Cathode Materials for Solid Oxide Fuel Cells. Ceram. Int. 2020, 46, 5521–5535. [Google Scholar] [CrossRef]
- Jun, A.; Kim, J.; Shin, J.; Kim, G. Perovskite as a Cathode Material: A Review of Its Role in Solid-Oxide Fuel Cell Technology. ChemElectroChem 2016, 3, 511–530. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A.; Kharytonau, D.S.; Medvedev, D.A. Layered oxygen-deficient double perovskites as promising cathode materials for solid oxide fuel cells. Materials 2022, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Vinoth Kumar, R.; Khandale, A.P. A Review on Recent Progress and Selection of Cobalt-Based Cathode Materials for Low Temperature-Solid Oxide Fuel Cells. Renew. Sustain. Energy Rev. 2022, 156, 111985. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of Lanthanum Strontium Manganite Perovskite Cathode Materials of Solid Oxide Fuel Cells: A Review. J. Mater. Sci. 2008, 43, 6799–6833. [Google Scholar] [CrossRef]
- Carda, M.; Budáč, D.; Paidar, M.; Bouzek, K. Current Trends in the Description of Lanthanum Strontium Manganite Oxygen Electrode Reaction Mechanism in a High-Temperature Solid Oxide Cell. Curr. Opin. Electrochem. 2022, 31, 100852. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Venâncio, R.; de Azevedo, P.V.; Anchieta, C.G.; Nepel, T.C.; Rodella, C.B.; Zanin, H.; Doubek, G. Reviewing Perovskite Oxide Sites Influence on Electrocatalytic Reactions for High Energy Density Devices. J. Energy Chem. 2023, 81, 1–19. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of Lanthanum Strontium Cobalt Ferrite Perovskite Electrodes of Solid Oxide Fuel Cells—A Review. Int. J. Hydrogen Energy 2019, 44, 7448–7493. [Google Scholar] [CrossRef]
- Ndubuisi, A.; Abouali, S.; Singh, K.; Thangadurai, V. Recent Advances, Practical Challenges, and Perspectives of Intermediate Temperature Solid Oxide Fuel Cell Cathodes. J. Mater. Chem. A 2022, 10, 2196–2227. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zhu, Z. Perovskite Cathode Materials for Low-Temperature Solid Oxide Fuel Cells: Fundamentals to Optimization. Electrochem. Energy Rev. 2022, 5, 263–311. [Google Scholar] [CrossRef]
- Safian, S.D.; Abd Malek, N.I.; Jamil, Z.; Lee, S.-W.; Tseng, C.-J.; Osman, N. Study on the Surface Segregation of Mixed Ionic-Electronic Conductor Lanthanum-Based Perovskite Oxide La1 − xSrxCo1 − yFeyO3 − δ Materials. Int. J. Energy Res. 2022, 46, 7101–7117. [Google Scholar] [CrossRef]
- Zarabi Golkhatmi, S.; Asghar, M.I.; Lund, P.D. A Review on Solid Oxide Fuel Cell Durability: Latest Progress, Mechanisms, and Study Tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Connor, P.A.; Yue, X.; Savaniu, C.D.; Price, R.; Triantafyllou, G.; Cassidy, M.; Kerherve, G.; Payne, D.J.; Maher, R.C.; Cohen, L.F.; et al. Tailoring SOFC Electrode Microstructures for Improved Performance. Adv. Energy Mater. 2018, 8, 1800120. [Google Scholar] [CrossRef]
- Celik, I.; Lee, S.; Abernathy, H.; Hackett, G. Performance Degradation Predictions Based on Microstructural Evolution Due to Grain Coarsening Effects in Solid Oxide Fuel Cell Electrodes. J. Electrochem. Soc. 2018, 165, F64–F74. [Google Scholar] [CrossRef]
- Geofrey Sahini, M.; Daud Lupyana, S. Perspective and Control of Cation Interdiffusion and Interface Reactions in Solid Oxide Fuel Cells (SOFCs). Mater. Sci. Eng. B 2023, 292, 116415. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, G.; Dai, R.; Lv, X.; Yang, D.; Geng, S. A Review of the Chemical Compatibility between Oxide Electrodes and Electrolytes in Solid Oxide Fuel Cells. J. Power Sources 2021, 492, 229630. [Google Scholar] [CrossRef]
- Khan, M.Z.; Song, R.-H.; Mehran, M.T.; Lee, S.-B.; Lim, T.-H. Controlling Cation Migration and Inter-Diffusion across Cathode/Interlayer/Electrolyte Interfaces of Solid Oxide Fuel Cells: A Review. Ceram. Int. 2021, 47, 5839–5869. [Google Scholar] [CrossRef]
- Wang, F.; Kishimoto, H.; Ishiyama, T.; Develos-Bagarinao, K.; Yamaji, K.; Horita, T.; Yokokawa, H. A Review of Sulfur Poisoning of Solid Oxide Fuel Cell Cathode Materials for Solid Oxide Fuel Cells. J. Power Sources 2020, 478, 228763. [Google Scholar] [CrossRef]
- Wang, C.C.; O’Donnell, K.; Jian, L.; Jiang, S.P. Co-Deposition and Poisoning of Chromium and Sulfur Contaminants on La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes of Solid Oxide Fuel Cells. J. Electrochem. Soc. 2015, 162, F507–F512. [Google Scholar] [CrossRef]
- Wang, R.; Parent, L.R.; Gopalan, S.; Zhong, Y. Experimental and Computational Investigations on the SO2 Poisoning of (La0.8Sr0.2)0.95MnO3 Cathode Materials. Adv. Powder Mater. 2023, 2, 100062. [Google Scholar] [CrossRef]
- Horita, T. Chromium Poisoning for Prolonged Lifetime of Electrodes in Solid Oxide Fuel Cells—Review. Ceram. Int. 2021, 47, 7293–7306. [Google Scholar] [CrossRef]
- Zhou, L.; Mason, J.H.; Li, W.; Liu, X. Comprehensive Review of Chromium Deposition and Poisoning of Solid Oxide Fuel Cells (SOFCs) Cathode Materials. Renew. Sustain. Energy Rev. 2020, 134, 110320. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Z.; Lu, Y.; Gopalan, S.; Basu, S.N.; Pal, U.B. Comparison of Chromium Poisoning between Lanthanum Strontium Manganite and Lanthanum Strontium Ferrite Composite Cathodes in Solid Oxide Fuel Cells. J. Power Sources 2020, 476, 228743. [Google Scholar] [CrossRef]
- Jiang, S.P.; Chen, X. Chromium Deposition and Poisoning of Cathodes of Solid Oxide Fuel Cells—A Review. Int. J. Hydrogen Energy 2014, 39, 505–531. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Jia, L.; Chi, B.; Pu, J.; Li, J. High Performance and Carbon-Deposition Resistance Metal-Supported Solid Oxide Fuel Cell with a Nickel–Manganese Spinel Modified Anode. Mater. Today Energy 2020, 17, 100473. [Google Scholar] [CrossRef]
- Girona, K.; Laurencin, J.; Fouletier, J.; Lefebvre-Joud, F. Carbon Deposition in CH4/CO2 Operated SOFC: Simulation and Experimentation Studies. J. Power Sources 2012, 210, 381–391. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, S.P. Surface Segregation in Solid Oxide Cell Oxygen Electrodes: Phenomena, Mitigation Strategies and Electrochemical Properties. Electrochem. Energy Rev. 2020, 3, 730–765. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Yao, C.; Lou, H.; Chen, M.; Lang, X.; Cai, K. Review of SOFC Cathode Performance Enhancement by Surface Modifications: Recent Advances and Future Directions. Energy Fuels 2023, 37, 3470–3487. [Google Scholar] [CrossRef]
- Jiang, S.P. Issues on Development of (La,Sr)MnO3 Cathode for Solid Oxide Fuel Cells. J. Power Sources 2003, 124, 390–402. [Google Scholar] [CrossRef]
- Cherepanov, V.A.; Barkhatova, L.Y.; Voronin, V.I. Phase Equilibria in the La–Sr–Mn–O System. J. Solid State Chem. 1997, 134, 38–44. [Google Scholar] [CrossRef]
- Cherepanov, V.A.; Filonova, E.A.; Voronin, V.I.; Berger, I.F.; Barkhatova, L.Y. Phase Equilibria in the LaCoO3–LaMnO3–SrCoO2.5–SrMnO3 System. Mater. Res. Bull. 1999, 34, 1481–1489. [Google Scholar] [CrossRef]
- Filonova, E.A.; Demina, A.N.; Kleibaum, E.A.; Gavrilova, L.Y.; Petrov, A.N. Phase Equilibria in the System LaMnO3+δ-SrMnO3-LaFeO3-SrFeO3 − d. Inorg. Mater. 2006, 42, 443–447. [Google Scholar] [CrossRef]
- Yusenko, M.V.; Belyaev, V.D.; Demin, A.K.; Bronin, D.I.; Sobyanin, V.A.; Snytnikov, P.V. A Study of the Electrochemical Characteristics of Single-Chamber Solid Oxide Fuel Cells Based on Platinum and Strontium-Doped Lanthanum Manganite Electrodes and Fed with a Methane–Air Mixture. Kinet. Catal. 2022, 63, 117–122. [Google Scholar] [CrossRef]
- Mizusaki, J. Electronic Conductivity, Seebeck Coefficient, Defect and Electronic Structure of Nonstoichiometric La1 − xSrxMnO3. Solid State Ion. 2000, 132, 167–180. [Google Scholar] [CrossRef]
- Mori, M.; Hiei, Y.; Sammes, N.M.; Tompsett, G.A. Thermal-Expansion Behaviors and Mechanisms for Ca- or Sr-Doped Lanthanum Manganite Perovskites under Oxidizing Atmospheres. J. Electrochem. Soc. 2000, 147, 1295. [Google Scholar] [CrossRef]
- Demina, A.N.; Polovnikova, K.P.; Filonova, E.A.; Petrov, A.N.; Demin, A.K.; Pikalova, E.Y. Thermal Expansion and Electrical Conductivity of La0.7Sr0.3Mn1 − yCryO3. Inorg. Mater. 2007, 43, 430–435. [Google Scholar] [CrossRef]
- Løken, A.; Ricote, S.; Wachowski, S. Thermal and Chemical Expansion in Proton Ceramic Electrolytes and Compatible Electrodes. Crystals 2018, 8, 365. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Sereda, V.V.; Malyshkin, D.A.; Ivanov, I.L.; Zuev, A.Y. Chemical Lattice Strain in Nonstoichiometric Oxides: An Overview. J. Mater. Chem. A 2022, 10, 6351–6375. [Google Scholar] [CrossRef]
- Maiti, T.K.; Majhi, J.; Maiti, S.K.; Singh, J.; Dixit, P.; Rohilla, T.; Ghosh, S.; Bhushan, S.; Chattopadhyay, S. Zirconia- and Ceria-Based Electrolytes for Fuel Cell Applications: Critical Advancements toward Sustainable and Clean Energy Production. Environ. Sci. Pollut. Res. 2022, 29, 64489–64512. [Google Scholar] [CrossRef] [PubMed]
- Sameshima, S.; Kawaminami, M.; Hirata, Y. Thermal Expansion of Rare-Earth-Doped Ceria Ceramics. J. Ceram. Soc. Jpn. 2002, 110, 597–600. [Google Scholar] [CrossRef]
- Tsvinkinberg, V.A.; Tolkacheva, A.S.; Filonova, E.A.; Gyrdasova, O.I.; Pikalov, S.M.; Vorotnikov, V.A.; Vylkov, A.I.; Moskalenko, N.I.; Pikalova, E.Y. Structure, Thermal Expansion and Electrical Conductivity of La2 − xGdxNiO4+δ (0.0 ≤ x ≤ 0.6) Cathode Materials for SOFC Applications. J. Alloys Compd. 2021, 853, 156728. [Google Scholar] [CrossRef]
- Lee, D.; Han, J.-H.; Chun, Y.; Song, R.-H.; Shin, D.R. Preparation and Characterization of Strontium and Magnesium Doped Lanthanum Gallates as the Electrolyte for IT-SOFC. J. Power Sources 2007, 166, 35–40. [Google Scholar] [CrossRef]
- Tietz, F. Thermal Expansion of SOFC Materials. Ionics 1999, 5, 129–139. [Google Scholar] [CrossRef]
- De Haart, L.G.J.; Vinke, I.C. Long-Term Operation of Planar Type SOFC Stacks. ECS Trans. 2011, 35, 187–194. [Google Scholar] [CrossRef]
- Kim-Lohsoontorn, P.; Brett, D.J.L.; Laosiripojana, N.; Kim, Y.-M.; Bae, J.-M. Performance of Solid Oxide Electrolysis Cells Based on Composite La0.8Sr0.2MnO3 − δ—Yttria Stabilized Zirconia and Ba0.5Sr0.5Co0.8Fe0.2O3 − δ Oxygen Electrodes. Int. J. Hydrogen Energy 2010, 35, 3958–3966. [Google Scholar] [CrossRef]
- De Souza, R.A.; Kilner, J.A.; Walker, J.F. A SIMS Study of Oxygen Tracer Diffusion and Surface Exchange in La0.8Sr0.2MnO3+δ. Mater. Lett. 2000, 43, 43–52. [Google Scholar] [CrossRef]
- Tai, L. Structure and Electrical Properties of La1 − xSrxCo1 − yFeyO3. Part 2. The System La1 − xSrxCo0.2Fe0.8O3. Solid State Ion. 1995, 76, 273–283. [Google Scholar] [CrossRef]
- Mineshige, A.; Izutsu, J.; Nakamura, M.; Nigaki, K.; Kobune, M.; Fujii, S.; Inaba, M.; Ogumi, Z.; Yao, T. Electrical Property, Crystal Structure and Oxygen Nonstoichiometry of La1 − xSrxCo0.2Fe0.8O3 − δ. Electrochemistry 2000, 68, 515–518. [Google Scholar] [CrossRef]
- Teraoka, Y.; Zhang, H.M.; Okamoto, K.; Yamazoe, N. Mixed Ionic-Electronic Conductivity of La1 − xSrxCo1 − yFeyO3 − δ Perovskite-Type Oxides. Mater. Res. Bull. 1988, 23, 51–58. [Google Scholar] [CrossRef]
- Petric, A. Evaluation of La–Sr–Co–Fe–O Perovskites for Solid Oxide Fuel Cells and Gas Separation Membranes. Solid State Ion. 2000, 135, 719–725. [Google Scholar] [CrossRef]
- Carter, S. Oxygen Transport in Selected Nonstoichiometric Perovskite-Structure Oxides. Solid State Ion. 1992, 53–56, 597–605. [Google Scholar] [CrossRef]
- Katsuki, M. High Temperature Properties of La0.6Sr0.4Co0.8Fe0.2O3 − δ Oxygen Nonstoichiometry and Chemical Diffusion Constant. Solid State Ion. 2003, 156, 453–461. [Google Scholar] [CrossRef]
- Simner, S.P.; Anderson, M.D.; Engelhard, M.H.; Stevenson, J.W. Degradation Mechanisms of La–Sr–Co–Fe–O3 SOFC Cathodes. Electrochem. Solid-State Lett. 2006, 9, A478. [Google Scholar] [CrossRef]
- Wang, H.; Barnett, S.A. Degradation Mechanisms of Porous La0.6Sr0.4Co0.2Fe0.8O3 − δ Solid Oxide Fuel Cell Cathodes. J. Electrochem. Soc. 2018, 165, F564–F570. [Google Scholar] [CrossRef]
- Laurencin, J.; Hubert, M.; Sanchez, D.F.; Pylypko, S.; Morales, M.; Morata, A.; Morel, B.; Montinaro, D.; Lefebvre-Joud, F.; Siebert, E. Degradation Mechanism of La0.6Sr0.4Co0.2Fe0.8O3 − δ/Gd0.1Ce0.9O2 − δ Composite Electrode Operated under Solid Oxide Electrolysis and Fuel Cell Conditions. Electrochim. Acta 2017, 241, 459–476. [Google Scholar] [CrossRef]
- Zhao, L.; Drennan, J.; Kong, C.; Amarasinghe, S.; Jiang, S.P. Insight into Surface Segregation and Chromium Deposition on La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes of Solid Oxide Fuel Cells. J. Mater. Chem. A 2014, 2, 11114–11123. [Google Scholar] [CrossRef]
- Lee, W.; Han, J.W.; Chen, Y.; Cai, Z.; Yildiz, B. Cation Size Mismatch and Charge Interactions Drive Dopant Segregation at the Surfaces of Manganite Perovskites. J. Am. Chem. Soc. 2013, 135, 7909–7925. [Google Scholar] [CrossRef]
- He, S.; Jiang, S.P. Electrode/Electrolyte Interface and Interface Reactions of Solid Oxide Cells: Recent Development and Advances. Prog. Nat. Sci. 2021, 31, 341–372. [Google Scholar] [CrossRef]
- Stochniol, G.; Syskakis, E.; Naoumidis, A. Chemical Compatibility between Strontium-Doped Lanthanum Manganite and Yttria-Stabilized Zirconia. J. Am. Ceram. Soc. 1995, 78, 929–932. [Google Scholar] [CrossRef]
- Yokokawa, H.; Tu, H.; Iwanschitz, B.; Mai, A. Fundamental Mechanisms Limiting Solid Oxide Fuel Cell Durability. J. Power Sources 2008, 182, 400–412. [Google Scholar] [CrossRef]
- Kindermann, L. Chemical Compatibility of the LaFeO3 Base Perovskites (La0.6Sr0.4)ZFe0.8M0.2O3 − δ (z = 1, 0.9; M = Cr, Mn, Co, Ni) with Yttria Stabilized Zirconia. Solid State Ion. 1996, 89, 215–220. [Google Scholar] [CrossRef]
- Hubert, M.; Laurencin, J.; Cloetens, P.; Mougin, J.; Ferreira Sanchez, D.; Pylypko, S.; Morales, M.; Morata, A.; Morel, B.; Montinaro, D.; et al. Solid Oxide Cell Degradation Operated in Fuel Cell and Electrolysis Modes: A Comparative Study on Ni Agglomeration and LSCF Destabilization. ECS Trans. 2017, 78, 3167–3177. [Google Scholar] [CrossRef]
- Kostogloudis, G. Chemical Reactivity of Perovskite Oxide SOFC Cathodes and Yttria Stabilized Zirconia. Solid State Ion. 2000, 135, 529–535. [Google Scholar] [CrossRef]
- Fan, B.; Yan, J.; Yan, X. The Ionic Conductivity, Thermal Expansion Behavior, and Chemical Compatibility of La0.54Sr0.44Co0.2Fe0.8O3 − δ as SOFC Cathode Material. Solid State Sci. 2011, 13, 1835–1839. [Google Scholar] [CrossRef]
- Darvish, S.; Asadikiya, M.; Hu, B.; Singh, P.; Zhong, Y. Thermodynamic Prediction of the Effect of CO2 to the Stability of (La0.8Sr0.2)0.98MnO3±δ System. Int. J. Hydrogen Energy 2016, 41, 10239–10248. [Google Scholar] [CrossRef]
- Hu, B.; Mahapatra, M.K.; Keane, M.; Zhang, H.; Singh, P. Effect of CO2 on the Stability of Strontium Doped Lanthanum Manganite Cathode. J. Power Sources 2014, 268, 404–413. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Zhang, X.; Wu, W.; Tu, B.; Ou, D.; Cheng, M. A Comparison on Effects of CO2 on La0.8Sr0.2MnO3+δ and La0.6Sr0.4CoO3 − δ Cathodes. J. Power Sources 2013, 222, 542–553. [Google Scholar] [CrossRef]
- Darvish, S.; Gopalan, S.; Zhong, Y. Thermodynamic Stability Maps for the La0.6Sr0.4Co0.2Fe0.8O3±δ–CO2–O2 System for Application in Solid Oxide Fuel Cells. J. Power Sources 2016, 336, 351–359. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Zhang, X.; Wu, W.; Tu, B.; Cui, D.; Ou, D.; Cheng, M. High- and Low- Temperature Behaviors of La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathode Operating under CO2/H2O-Containing Atmosphere. Int. J. Hydrogen Energy 2013, 38, 15361–15370. [Google Scholar] [CrossRef]
- Lai, S.Y.; Ding, D.; Liu, M.; Liu, M.; Alamgir, F.M. Operando and in Situ X-ray Spectroscopies of Degradation in La0.6Sr0.4Co0.2Fe0.8O3 − δ Thin Film Cathodes in Fuel Cells. ChemSusChem 2014, 7, 3078–3087. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hagen, A.; Barfod, R.; Chen, M.; Wang, H.J.; Poulsen, F.W.; Hendriksen, P.V. Microstructural Studies on Degradation of Interface between LSM–YSZ Cathode and YSZ Electrolyte in SOFCs. Solid State Ion. 2009, 180, 1298–1304. [Google Scholar] [CrossRef]
- Hu, B.; Mahapatra, M.K.; Singh, P. Performance Regeneration in Lanthanum Strontium Manganite Cathode during Exposure to H2O and CO2 Containing Ambient Air Atmospheres. J. Ceram. Soc. Jpn. 2015, 123, 199–204. [Google Scholar] [CrossRef]
- Xia, Z.; Zhao, D.; Zhou, Y.; Deng, Z.; Kupecki, J.; Fu, X.; Li, X. Control-Oriented Performance Prediction of Solid Oxide Electrolysis Cell and Durability Improvement through Retard Oxygen Electrode Delamination with Reverse Operation. Energy Convers. Manag. 2023, 277, 116596. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Huang, Y.-L.; Pellegrinelli, C.; Taillon, J.A.; Salamanca-Riba, L.G. Towards a Fundamental Understanding of the Cathode Degradation Mechanisms. ECS Trans. 2014, 61, 47–56. [Google Scholar] [CrossRef]
- Wei, B.; Chen, K.; Zhao, L.; Lü, Z.; Jiang, S.P. Chromium Deposition and Poisoning at La0.6Sr0.4Co0.2Fe0.8O3 − δ Oxygen Electrodes of Solid Oxide Electrolysis Cells. Phys. Chem. Chem. Phys. 2015, 17, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Subotić, V.; Futamura, S.; Harrington, G.F.; Matsuda, J.; Natsukoshi, K.; Sasaki, K. Towards Understanding of Oxygen Electrode Processes during Solid Oxide Electrolysis Operation to Improve Simultaneous Fuel and Oxygen Generation. J. Power Sources 2021, 492, 229600. [Google Scholar] [CrossRef]
- He, S.; Saunders, M.; Chen, K.; Gao, H.; Suvorova, A.; Rickard, W.D.A.; Quadir, Z.; Cui, C.Q.; Jiang, S.P. A FIB-STEM Study of Strontium Segregation and Interface Formation of Directly Assembled La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathode on Y2O3-ZrO2 Electrolyte of Solid Oxide Fuel Cells. J. Electrochem. Soc. 2018, 165, F417–F429. [Google Scholar] [CrossRef]
- Pellegrinelli, C.; Huang, Y.-L.; Wachsman, E.D. Effect of H2O and CO2 on LSCF−GDC Composite Cathodes. ECS Trans. 2019, 91, 665–680. [Google Scholar] [CrossRef]
- Xiong, C.; Qiu, P.; Zhang, W.; Pu, J. Influence of Practical Operating Temperature on the Cr Poisoning for LSCF-GDC Cathode. Ceram. Int. 2022, 48, 33999–34004. [Google Scholar] [CrossRef]
- Minh, N.Q. Development of Reversible Solid Oxide Fuel Cells (RSOFCs)and Stacks. ECS Trans. 2011, 35, 2897–2904. [Google Scholar] [CrossRef]
- Monaco, F.; Ferreira-Sanchez, D.; Hubert, M.; Morel, B.; Montinaro, D.; Grolimund, D.; Laurencin, J. Oxygen Electrode Degradation in Solid Oxide Cells Operating in Electrolysis and Fuel Cell Modes: LSCF Destabilization and Interdiffusion at the Electrode/Electrolyte Interface. Int. J. Hydrogen Energy 2021, 46, 31533–31549. [Google Scholar] [CrossRef]
- Lu, K.; Shen, F. Long Term Behaviors of La0.8Sr0.2MnO3 and La0.6Sr0.4Co0.2Fe0.8O3 as Cathodes for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2014, 39, 7963–7971. [Google Scholar] [CrossRef]
- Türk, H.; Götsch, T.; Schmidt, F.; Hammud, A.; Ivanov, D.; (Bert) de Haart, L.G.J.; Vinke, I.C.; Eichel, R.; Schlögl, R.; Reuter, K.; et al. Sr Surface Enrichment in Solid Oxide Cells—Approaching the Limits of EDX Analysis by Multivariate Statistical Analysis and Simulations. ChemCatChem 2022, 14, e202200300. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Liang, C.; Jin, Y.; Fu, M.; Yuan, J.; Xiong, Y. Study on Durability of Novel Core-Shell-Structured La0.8Sr0.2Co0.2Fe0.8O3 − δ@Gd0.2Ce0.8O1.9 Composite Materials for Solid Oxide Fuel Cell Cathodes. Int. J. Hydrogen Energy 2021, 46, 28221–28231. [Google Scholar] [CrossRef]
- Türk, H.; Schmidt, F.; Götsch, T.; Girgsdies, F.; Hammud, A.; Ivanov, D.; Vinke, I.C.; (Bert) de Haart, L.G.J.; Eichel, R.; Reuter, K.; et al. Complexions at the Electrolyte/Electrode Interface in Solid Oxide Cells. Adv. Mater. Interfaces 2021, 8, 2100967. [Google Scholar] [CrossRef]
- Cheng, K.; Xu, H.; Zhang, L.; Du, Y.; Zhou, J.; Tang, S.; Chen, M. Numerical Simulation of the SrZrO3 Formation in Solid Oxide Fuel Cells. J. Electron. Mater. 2019, 48, 5510–5515. [Google Scholar] [CrossRef]
- Jacobs, R.; Liu, J.; Na, B.T.; Guan, B.; Yang, T.; Lee, S.; Hackett, G.; Kalapos, T.; Abernathy, H.; Morgan, D. Unconventional Highly Active and Stable Oxygen Reduction Catalysts Informed by Computational Design Strategies. Adv. Energy Mater. 2022, 12, 2201203. [Google Scholar] [CrossRef]
- Han, H.; Hu, X.; Zhang, B.; Zhang, S.; Zhang, Y.; Xia, C. Method to Determine the Oxygen Reduction Reaction Kinetics via Porous Dual-Phase Composites Based on Electrical Conductivity Relaxation. J. Mater. Chem. A 2023, 11, 2460–2471. [Google Scholar] [CrossRef]
- Yan, Z.; He, A.; Hara, S.; Shikazono, N. Design and Optimization of Functionally Graded Electrodes for Solid Oxide Fuel Cells (SOFCs) by Mesoscale Modeling. Int. J. Hydrogen Energy 2022, 47, 16610–16625. [Google Scholar] [CrossRef]
- Padinjarethil, A.K.; Bianchi, F.R.; Bosio, B.; Hagen, A. Electrochemical Characterization and Modelling of Anode and Electrolyte Supported Solid Oxide Fuel Cells. Front. Energy Res. 2021, 9, 668964. [Google Scholar] [CrossRef]
- Bliem, R.; Kim, D.; Wang, J.; Crumlin, E.J.; Yildiz, B. Hf Deposition Stabilizes the Surface Chemistry of Perovskite Manganite Oxide. J. Phys. Chem. C 2021, 125, 3346–3354. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Xu, K.; Zhu, F.; Chen, Y. Enhancing the Oxygen Reduction Reaction Activity and Durability of a Solid Oxide Fuel Cell Cathode by Surface Modification of a Hybrid Coating. Int. J. Hydrogen Energy 2023, 48, 23992–24001. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Wu, C.; Zhao, L.; Dong, B.; Wang, S.; Chi, B. Self-Stabilized Hybrid Cathode for Solid Oxide Fuel Cell: A-Site Deficient Perovskite Coating as Solid Solution for Strontium Diffusion. Chem. Eng. J. 2022, 438, 135446. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Y.; Jiang, Y.; Zong, X.; Li, Y.; Xiong, Y. Enhancing Chromium Poisoning Tolerance of La0.8Sr0.2Co0.2Fe0.8O3 − δ Cathode by Ce0.8Gd0.2O1.9 − δ Coating. J. Power Sources 2022, 547, 231996. [Google Scholar] [CrossRef]
- Pei, K.; Zhou, Y.; Ding, Y.; Xu, K.; Zhang, H.; Yuan, W.; Sasaki, K.; Choi, Y.; Liu, M.; Chen, Y. An Improved Oxygen Reduction Reaction Activity and CO2-Tolerance of La0.6Sr0.4Co0.2Fe0.8O3 − δ Achieved by a Surface Modification with Barium Cobaltite Coatings. J. Power Sources 2021, 514, 230573. [Google Scholar] [CrossRef]
- Pei, K.; Zhou, Y.; Xu, K.; He, Z.; Chen, Y.; Zhang, W.; Yoo, S.; Zhao, B.; Yuan, W.; Liu, M.; et al. Enhanced Cr-Tolerance of an SOFC Cathode by an Efficient Electro-Catalyst Coating. Nano Energy 2020, 72, 104704. [Google Scholar] [CrossRef]
- Ishfaq, H.A.; Khan, M.Z.; Shirke, Y.M.; Qamar, S.; Hussain, A.; Mehran, M.T.; Song, R.-H.; Saleem, M. A Heuristic Approach to Boost the Performance and Cr Poisoning Tolerance of Solid Oxide Fuel Cell Cathode by Robust Multi-Doped Ceria Coating. Appl. Catal. B Environ. 2023, 323, 122178. [Google Scholar] [CrossRef]
- Chen, Y.; Hinerman, A.; Liang, L.; Gerdes, K.; Navia, S.P.; Prucz, J.; Song, X. Conformal Coating of Cobalt Oxide on Solid Oxide Fuel Cell Cathode and Resultant Continuously Increased Oxygen Reduction Reaction Kinetics upon Operation. J. Power Sources 2018, 405, 45–50. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, Y.; Zhang, W.; Zhang, Y.; Evans, C.; Luo, Z.; Kane, N.; Ding, Y.; Chen, Y.; Guo, X.; et al. Highly Active and Durable Air Electrodes for Reversible Protonic Ceramic Electrochemical Cells Enabled by an Efficient Bifunctional Catalyst. Adv. Energy Mater. 2022, 12, 2103783. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Y.; Li, D.; Zong, X.; Xiong, Y. Effects of Gd0.8Ce0.2O1.9 − δ Coating with Different Thickness on Electrochemical Performance and Long-Term Stability of La0.8Sr0.2Co0.2Fe0.8O3 − δ Cathode in SOFCs. Int. J. Hydrogen Energy 2022, 47, 4100–4108. [Google Scholar] [CrossRef]
- Chen, Y.; Paredes-Navia, S.A.; Romo-De-La-Cruz, C.-O.; Liang, L.; Fernandes, A.; Hinerman, A.; Prucz, J.; Williams, M.; Song, X. Coating Internal Surface of Porous Electrode for Decreasing the Ohmic Resistance and Shifting Oxygen Reduction Reaction Pathways in Solid Oxide Fuel Cells. J. Power Sources 2021, 499, 229854. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, Y.; Lv, W.; Chen, Y.; Zhang, Y.; Zhang, W.; Luo, Z.; Kane, N.; Ding, Y.; Soule, L.; et al. Enhancing Oxygen Reduction Activity and Cr Tolerance of Solid Oxide Fuel Cell Cathodes by a Multiphase Catalyst Coating. Adv. Funct. Mater. 2021, 31, 2100034. [Google Scholar] [CrossRef]
- Yokokawa, H.; Horita, T. Cathodes. In High Temperature and Solid Oxide Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2003; pp. 119–147. ISBN 978-1-85617-387-2. [Google Scholar]
- Filonova, E.A.; Gilev, A.R.; Skutina, L.S.; Vylkov, A.I.; Kuznetsov, D.K.; Shur, V.Y. Double Sr2Ni1 − xMgxMoO6 Perovskites (x = 0, 0.25) as Perspective Anode Materials for LaGaO3-Based Solid Oxide Fuel Cells. Solid State Ion. 2018, 314, 112–118. [Google Scholar] [CrossRef]
- Wu, Y.; Sang, J.; Liu, Z.; Fan, H.; Cao, B.; Wang, Q.; Yang, J.; Guan, W.; Liu, X.; Wang, J. Enhancing the Performance and Stability of Solid Oxide Fuel Cells by Adopting Samarium-Doped Ceria Buffer Layer. Ceram. Int. 2023, 49, 20290–20297. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Tian, D.; Wu, H.; Ding, Y.; Lu, X.; Chen, Y.; Lin, B. Enhancing Performance and Stability of Symmetrical Solid Oxide Fuel Cells via Quasi-Symmetrical Ceria-Based Buffer Layers. Ceram. Int. 2022, 48, 27509–27515. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, B.; Hao, X.; Wang, Y.; Liu, J.; Jiang, P.; He, T. Layered Oxygen-Deficient Double Perovskite GdBaFe2O5+δ as Electrode Material for Symmetrical Solid-Oxide Fuel Cells. Electrochim. Acta 2021, 370, 137807. [Google Scholar] [CrossRef]
- Niemczyk, A.; Zheng, K.; Cichy, K.; Berent, K.; Küster, K.; Starke, U.; Poudel, B.; Dabrowski, B.; Świerczek, K. High Cu Content LaNi1-xCuxO3 − δ Perovskites as Candidate Air Electrode Materials for Reversible Solid Oxide Cells. Int. J. Hydrogen Energy 2020, 45, 29449–29464. [Google Scholar] [CrossRef]
- Yang, X.; Li, R.; Yang, Y.; Wen, G.; Tian, D.; Lu, X.; Ding, Y.; Chen, Y.; Lin, B. Improving Stability and Electrochemical Performance of Ba0.5Sr0.5Co0.2Fe0.8O3 − δ Electrode for Symmetrical Solid Oxide Fuel Cells by Mo Doping. J. Alloys Compd. 2020, 831, 154711. [Google Scholar] [CrossRef]
- Huang, K.; Wan, J.; Goodenough, J.B. Oxide-Ion Conducting Ceramics for Solid Oxide Fuel Cells. J. Mater. Sci. 2001, 36, 1093–1098. [Google Scholar] [CrossRef]
- Yun, J.W.; Yoon, S.P.; Park, S.; Han, J.; Nam, S.W.; Lim, T.-H.; Kim, J.-S. Modifying the Cathodes of Intermediate-Temperature Solid Oxide Fuel Cells with a Ce0.8Sm0.2O2 Sol–Gel Coating. Int. J. Hydrogen Energy 2009, 34, 9213–9219. [Google Scholar] [CrossRef]
- Hanifi, A.R.; Paulson, S.; Torabi, A.; Shinbine, A.; Tucker, M.C.; Birss, V.; Etsell, T.H.; Sarkar, P. Slip-Cast and Hot-Solution Infiltrated Porous Yttria Stabilized Zirconia (YSZ) Supported Tubular Fuel Cells. J. Power Sources 2014, 266, 121–131. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.; Kang, G.; Choi, M.; Kim, S.J.; Lee, W.; Byun, D. A Hydrogel-Assisted GDC Chemical Diffusion Barrier for Durable Solid Oxide Fuel Cells. J. Mater. Chem. A 2021, 9, 11683–11690. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Wang, Y.; Li, Y.; Pu, J.; Ciucci, F.; Chi, B. Nano Film Pr2Ni0.8Cu0.2O4+δ Decorated La0.6Sr0.4Co0.2Fe0.8O3 − δ Oxygen Electrode for Highly Efficient and Stable Reversible Solid Oxide Cells. Electrochim. Acta 2022, 430, 141032. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, N.; Dogdibegovic, E.; Su, T.; Brocato, A.D.; Zhou, X.-D. Role of Mixed Conducting Pr0.1Gd0.1Ce0.8O1.9 − δ Barrier Layer on the Promotion of SOFC Performance. Int. J. Hydrogen Energy 2022, 47, 1917–1924. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, C.H.; Kim, K.J.; Kim, D.; Shin, H.R.; Hong, J.-E.; Lee, K.T. Enhancing Bifunctional Electrocatalytic Activities of Oxygen Electrodes via Incorporating Highly Conductive Sm3+ and Nd3+ Double-Doped Ceria for Reversible Solid Oxide Cells. ACS Appl. Mater. Interfaces 2021, 13, 2496–2506. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Han, S.M.; Kim, B.-K.; Lee, J.-H.; Yoon, K.J.; Kim, H.; Ji, H.-I.; Son, J.-W. Sintered Powder-Base Cathode over Vacuum-Deposited Thin-Film Electrolyte of Low-Temperature Solid Oxide Fuel Cell: Performance and Stability. Electrochim. Acta 2019, 296, 1055–1063. [Google Scholar] [CrossRef]
- Choi, H.-J.; Na, Y.-H.; Kwak, M.; Kim, T.W.; Seo, D.-W.; Woo, S.-K.; Kim, S.-D. Development of Solid Oxide Cells by Co-Sintering of GDC Diffusion Barriers with LSCF Air Electrode. Ceram. Int. 2017, 43, 13653–13660. [Google Scholar] [CrossRef]
- Yoo, Y.-S.; Jeon, S.-Y.; Park, M.-A.; Lee, J.; Lee, Y. Electrochemical Performance of Solid Oxide Electrolysis Cells with LSCF6428–SDC/SDC Electrode for H2O/CO2 High Temperature Co-Electrolysis. ECS Trans. 2017, 78, 3123–3128. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, G.; Huang, Z.; Bao, X.; Shi, C.; Yang, X.; Zhou, J.; Chen, T.; Wang, S. The Effect of Fe2O3 Sintering Aid on Gd0.1Ce0.9O1.95 Diffusion Barrier Layer and Solid Oxide Fuel Cell Performance. Int. J. Hydrogen Energy 2023, 48, 21908–21919. [Google Scholar] [CrossRef]
- Budiman, R.A.; Yamaguchi, T.; Ishiyama, T.; Develos-Bagarinao, K.; Yamaji, K.; Kishimoto, H. Interlayer Modification for High-Performance and Stable Solid Oxide Electrolysis Cell. Mater. Lett. 2022, 309, 131419. [Google Scholar] [CrossRef]
- Wu, P.; Tian, Y.; Lü, Z.; Zhang, X.; Ding, L. Electrochemical Performance of La0.65Sr0.35MnO3 Oxygen Electrode with Alternately Infiltrated Sm0.5Sr0.5CoO3 − δ and Sm0.2Ce0.8O1.9 Nanoparticles for Reversible Solid Oxide Cells. Int. J. Hydrogen Energy 2022, 47, 747–760. [Google Scholar] [CrossRef]
- Yaroslavtsev, I.Y.; Kuzin, B.L.; Bronin, D.I.; Bogdanovich, N.M. Polarization Characteristics of Composite Electrodes in Electrochemical Cells with Solid Electrolytes Based on CeO2 and LaGaO3. Russ. J. Electrochem. 2005, 41, 527–531. [Google Scholar] [CrossRef]
- Mosiałek, M.; Zimowska, M.; Kharytonau, D.; Komenda, A.; Górski, M.; Krzan, M. Improvement of La0.8Sr0.2MnO3 − δ Cathode Material for Solid Oxide Fuel Cells by Addition of YFe0.5Co0.5O3. Materials 2022, 15, 642. [Google Scholar] [CrossRef]
- Bogdanovich, N.M.; Bronin, D.I.; Vdovin, G.K.; Yaroslavtsev, I.Y.; Kuzin, B.L. Effect of Bi0.75Y0.25O1.5 Electrolyte Additive in Collector Layer to Properties of Bilayer Composite Cathodes of Solid Oxide Fuel Cells Based on La(Sr)MnO3 and La(Sr)Fe(Co)O3 Compounds. Russ. J. Electrochem. 2009, 45, 456–464. [Google Scholar] [CrossRef]
- Perry Murray, E. (La,Sr)MnO3–(Ce,Gd)O2 − x Composite Cathodes for Solid Oxide Fuel Cells. Solid State Ion. 2001, 143, 265–273. [Google Scholar] [CrossRef]
- Chen, G.; Gao, Y.; Luo, Y.; Guo, R. Effect of A Site Deficiency of LSM Cathode on the Electrochemical Performance of SOFCs with Stabilized Zirconia Electrolyte. Ceram. Int. 2017, 43, 1304–1309. [Google Scholar] [CrossRef]
- Sun, K.; Yu, Z.; Ni, Q.; Li, Y.; Xu, D.; Gu, Y.; Zheng, Y.; Chen, H.; Ge, L.; Guo, L. Highly Durable Sr-Doped LaMnO3-Based Cathode Modified with Pr6O11 Nano-Catalyst for Protonic Ceramic Fuel Cells Based on Y-Doped BaZrO3 Electrolyte. J. Eur. Ceram. Soc. 2022, 42, 4266–4274. [Google Scholar] [CrossRef]
- Cao, X.G.; Jiang, S.P. Identification of Oxygen Reduction Processes at (La,Sr)MnO3 Electrode/La9.5Si6O26.25 Apatite Electrolyte Interface of Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2013, 38, 2421–2431. [Google Scholar] [CrossRef]
- Strandbakke, R.; Dyrlie, O.; Hage, F.S.; Norby, T. Reaction Kinetics of Protons and Oxide Ions in LSM/Lanthanum Tungstate Cathodes with Pt Nanoparticle Activation. J. Electrochem. Soc. 2016, 163, F507–F515. [Google Scholar] [CrossRef]
- Shen, F.; Wang, R.; Tucker, M.C. Long Term Durability Test and Post Mortem for Metal-Supported Solid Oxide Electrolysis Cells. J. Power Sources 2020, 474, 228618. [Google Scholar] [CrossRef]
- Mater, A.; Othmani, A.; Boukhachem, A.; Madani, A. Cathode Performance Study of La0.6Sr0.4Co0.8Fe0.2O3 − δ with Various Electrolyte-Doped Ceria Ce0.8Sm0.17Ln0.03O1.9 for IT-Solid Oxide Fuel Cell. J. Electron. Mater. 2020, 49, 4123–4133. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, J.G.; He, H.G.; Gao, J.F. Ni/SDC Materials for Solid Oxide Fuel Cell Anode Applications by the Glycine-Nitrate Method. Key Eng. Mater. 2010, 434–435, 731–734. [Google Scholar] [CrossRef]
- Ahuja, A.; Gautam, M.; Sinha, A.; Sharma, J.; Patro, P.K.; Venkatasubramanian, A. Effect of Processing Route on the Properties of LSCF-Based Composite Cathode for IT-SOFC. Bull. Mater. Sci. 2020, 43, 129. [Google Scholar] [CrossRef]
- He, A.; Onishi, J.; Shikazono, N. Optimization of Electrode-Electrolyte Interface Structure for Solid Oxide Fuel Cell Cathode. J. Power Sources 2020, 449, 227565. [Google Scholar] [CrossRef]
- Loureiro, F.J.A.; Macedo, D.A.; Nascimento, R.M.; Cesário, M.R.; Grilo, J.P.F.; Yaremchenko, A.A.; Fagg, D.P. Cathodic Polarisation of Composite LSCF-SDC IT-SOFC Electrode Synthesised by One-Step Microwave Self-Assisted Combustion. J. Eur. Ceram. Soc. 2019, 39, 1846–1853. [Google Scholar] [CrossRef]
- Wei, F.; Wang, L.; Luo, L.; Cheng, L.; Xu, X. One-Pot Impregnation to Construct Nanoparticles Loaded Scaffold Cathode with Enhanced Oxygen Reduction Performance for LT-SOFCs. J. Alloys Compd. 2023, 941, 168981. [Google Scholar] [CrossRef]
- Sun, Y.; He, S.; Saunders, M.; Chen, K.; Shao, Z.; Jiang, S.P. A Comparative Study of Surface Segregation and Interface of La0.6Sr0.4Co0.2Fe0.8O3 − δ Electrode on GDC and YSZ Electrolytes of Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 2606–2616. [Google Scholar] [CrossRef]
- Abarzua, G.; Udayabhaskar, R.; Mangalaraja, R.V.; Durango-Petro, J.; Usuba, J.; Flies, H. A Feasible Strategy for Tailoring Stable Spray-Coated Electrolyte Layer in Micro-Tubular Solid Oxide Fuel Cells. Int. J. Appl. Ceram. Technol. 2022, 19, 1389–1396. [Google Scholar] [CrossRef]
- Samreen, A.; Galvez-Sanchez, M.; Steinberger-Wilckens, R.; Arifin, N.A.; Saher, S.; Ali, S.; Qamar, A. Electrochemical Performance of Novel NGCO−LSCF Composite Cathode for Intermediate Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 21714–21721. [Google Scholar] [CrossRef]
- Hong, T.; Lee, S.; Ohodnicki, P.; Brinkman, K. A Highly Scalable Spray Coating Technique for Electrode Infiltration: Barium Carbonate Infiltrated La0.6Sr0.4Co0.2Fe0.8O3 − δ Perovskite Structured Electrocatalyst with Demonstrated Long Term Durability. Int. J. Hydrogen Energy 2017, 42, 24978–24988. [Google Scholar] [CrossRef]
- Tsuji, Y.; Amezawa, K.; Nakao, T.; Ina, T.; Kawada, T.; Yamamoto, K.; Uchimoto, Y.; Orikasa, Y. Investigation of Cathodic Reaction Mechanism in Solid Oxide Fuel Cells by Operando X-ray Absorption Spectroscopy. Electrochemistry 2020, 88, 560–565. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Zhou, J.; Zhou, D.; Zhu, X.; Meng, J. Effects of CoO and Bi2O3 Single/Dual Sintering Aids Doping on Structure and Properties of Ce0.8Nd0.2O1.9. Ceram. Int. 2020, 46, 22727–22732. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Liu, S.-H.; Zhang, H.-Y.; Li, C.-X.; Zhang, S.-L.; Yang, G.-J.; Li, C.-J. Structured La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathode with Large-Scale Vertical Cracks by Atmospheric Laminar Plasma Spraying for IT-SOFCs. J. Alloys Compd. 2020, 825, 153865. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Yang, K.; Peng, S. Kinetics of Oxygen Reaction in Porous La0.6Sr0.4Co0.2Fe0.8O3 − δ-Ce0.8Gd0.2O1.9 Composite Electrodes for Solid Oxide Cells. Int. J. Hydrogen Energy 2021, 46, 25608–25619. [Google Scholar] [CrossRef]
- Khaerudini, D.S.; Guan, G.; Zhang, P.; Hao, X.; Wang, Z.; Xue, C.; Kasai, Y.; Abudula, A. Performance Assessment of Bi0.3Sr0.7Co0.3Fe0.7O3 − δ–LSCF Composite as Cathode for Intermediate-Temperature Solid Oxide Fuel Cells with La0.8Sr0.2Ga0.8Mg0.2O3 − δ Electrolyte. J. Power Sources 2015, 298, 269–279. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Shang, Y.-B.; Li, C.-X.; Li, C.-J. Vacuum Cold Sprayed Nanostructured La0.6Sr0.4Co0.2Fe0.8O3 − δ as a High-Performance Cathode for Porous Metal-Supported Solid Oxide Fuel Cells Operating below 600 °C. Mater. Today Energy 2021, 21, 100815. [Google Scholar] [CrossRef]
- Wang, S.-F.; Lu, H.-C.; Hsu, Y.-F.; Jasinski, P. High-Performance Anode-Supported Solid Oxide Fuel Cells with Co-Fired Sm0.2Ce0.8O2 − δ/La0.8Sr0.2Ga0.8Mg0.2O3 − δ/Sm0.2Ce0.8O2 − δ Sandwiched Electrolyte. Int. J. Hydrogen Energy 2022, 47, 5429–5438. [Google Scholar] [CrossRef]
- Lei, L.; Tao, Z.; Hong, T.; Wang, X.; Chen, F. A Highly Active Hybrid Catalyst Modified (La0.60Sr0.40)0.95Co0.20Fe0.80O3 − δ Cathode for Proton Conducting Solid Oxide Fuel Cells. J. Power Sources 2018, 389, 1–7. [Google Scholar] [CrossRef]
- Di Bartolomeo, E.; Zunic, M.; Chevallier, L.; D’Epifanio, A.; Licoccia, S.; Traversa, E. Fabrication of Proton Conducting Solid Oxide Fuel Cells by Using Electrophoretic Deposition. ECS Trans. 2009, 25, 577–584. [Google Scholar] [CrossRef]
- Watanabe, K.; Yamaguchi, Y.; Nomura, K.; Sumi, H.; Mori, M.; Mizutani, Y.; Shimada, H. Effect of Cobalt Content on Electrochemical Performance for La0.6Sr0.4CoxFe1 − xO3 − δ and BaZr0.8Yb0.2O3 − δ Composite Cathodes in Protonic Ceramic Fuel Cells. Ceram. Int. 2023, 49, 21085–21090. [Google Scholar] [CrossRef]
- Vafaeenezhad, S.; Sandhu, N.K.; Hanifi, A.R.; Etsell, T.H.; Sarkar, P. Development of Proton Conducting Fuel Cells Using Nickel Metal Support. J. Power Sources 2019, 435, 226763. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Y.; Chen, L.; Wang, L.; Chou, K. Enhancement of Electrochemical Performance for Proton Conductive Solid Oxide Fuel Cell by 30%GDC−LSCF Cathode. Ceram. Int. 2022, 48, 17816–17827. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Li, Y.; Xing, J.; Zhang, Z.; Ding, X.; Zhang, B.; Zhou, J.; Wang, S. Fabrication and Performance of Anode-Supported Proton Conducting Solid Oxide Fuel Cells Based on BaZr0.1Ce0.7Y0.1Yb0.1O3 − δ Electrolyte by Multi-Layer Aqueous-Based Co-Tape Casting. J. Power Sources 2021, 506, 229922. [Google Scholar] [CrossRef]
- Shimada, H.; Yamaguchi, Y.; Sumi, H.; Mizutani, Y. Performance Comparison of Perovskite Composite Cathodes with BaZr0.1Ce0.7Y0.1Yb0.1O3 − δ in Anode-Supported Protonic Ceramic Fuel Cells. J. Electrochem. Soc. 2020, 167, 124506. [Google Scholar] [CrossRef]
- Gao, J.; Meng, Y.; Lee, S.; Tong, J.; Brinkman, K.S. Effect of Infiltration of Barium Carbonate Nanoparticles on the Electrochemical Performance of La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes for Protonic Ceramic Fuel Cells. JOM 2019, 71, 90–95. [Google Scholar] [CrossRef]
- Lai, Y.-W.; Lee, K.-R.; Yang, S.-Y.; Tseng, C.-J.; Jang, S.-C.; Tsao, I.-Y.; Chen, S.; Lee, S.-W. Production of La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathode with Graded Porosity for Improving Proton-Conducting Solid Oxide Fuel Cells. Ceram. Int. 2019, 45, 22479–22485. [Google Scholar] [CrossRef]
- Fan, Y.; Xi, X.; Li, J.; Wang, Q.; Xiang, K.; Medvedev, D.; Luo, J.-L.; Fu, X.-Z. Barium-Doped Sr2Fe1.5Mo0.5O6 − δ Perovskite Anode Materials for Protonic Ceramic Fuel Cells for Ethane Conversion. J. Am. Ceram. Soc. 2022, 105, 3613–3624. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, Y.; Lin, X.; Fu, M.; Hu, W.; Tong, H.; Tao, Z. Investigation and Study of Three Different Composite Cathodes for Proton-Conducting Solid Oxide Fuel Cells. Sep. Purif. Technol. 2022, 300, 121890. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Lee, T.; Park, S.; Shin, D. Long Term Stability of Porosity Gradient Composite Cathode Controlled by Electro-Static Slurry Spray Deposition. Int. J. Hydrogen Energy 2017, 42, 3748–3752. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; Abadeen, Z.U.; Khan, K.; Tayyab, Z.; Qayyum, S.; Mosiałek, M.; Shao, Z.; Li, C.-X.; Motola, M. Proton-Conducting Solid Oxide Electrolysis Cells: Relationship of Composition-Structure-Property, Their Challenges, and Prospects. Matter 2023, 6, 1782–1830. [Google Scholar] [CrossRef]
- Zayas-Rey, M.J.; Dos Santos-Gómez, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Evaluation of Lanthanum Tungstates as Electrolytes for Proton Conductors Solid Oxide Fuel Cells. J. Power Sources 2015, 294, 483–493. [Google Scholar] [CrossRef]
- Marrero-López, D.; Martín-Sedeño, M.C.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Núñez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Evaluation of Apatite Silicates as Solid Oxide Fuel Cell Electrolytes. J. Power Sources 2010, 195, 2496–2506. [Google Scholar] [CrossRef]
- Nesaraj, A.S.; Kumar, M.; Arul Raj, I.; Radhakrishna, I.; Pattabiraman, R. Investigations on Chemical Interactions between Alternate Cathodes and Lanthanum Gallate Electrolyte for Intermediate Temperature Solid Oxide Fuel Cell (ITSOFC). J. Iran. Chem. Soc. 2007, 4, 89–106. [Google Scholar] [CrossRef]
- Giannici, F.; Chiara, A.; Canu, G.; Longo, A.; Martorana, A. Interface Solid-State Reactions in La0.8Sr0.2MnO3/Ce0.8Sm0.2O2 and La0.8Sr0.2MnO3/BaCe0.9Y0.1O3 Disclosed by X-ray Microspectroscopy. ACS Appl. Energy Mater. 2019, 2, 3204–3210. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Medvedev, D.; Pikalova, E.; Plaksin, S.; Brouzgou, A.; Demin, A.; Tsiakaras, P. A Detailed Analysis of Thermal and Chemical Compatibility of Cathode Materials Suitable for BaCe0.8Y0.2O3 − δ and BaZr0.8Y0.2O3 − δ Proton Electrolytes for Solid Oxide Fuel Cell Application. Int. J. Hydrogen Energy 2017, 42, 1715–1723. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Brouzgou, A.; Bronin, D.; Plaksin, S.V.; Khasanov, A.; Tsiakaras, P. Effect of Nature of the Ceramic Component of the Composite Electrodes Based on La1.7Ca(Sr)0.3NiO4+δ on Their Electrochemical Performance. ECS Trans. 2015, 68, 809–815. [Google Scholar] [CrossRef]
- Antonova, E.P.; Kolchugin, A.A.; Pikalova, E.Y.; Medvedev, D.A.; Bogdanovich, N.M. Development of Electrochemically Active Electrodes for BaCe0.89Gd0.1Cu0.01O3 − δ Proton Conducting Electrolyte. Solid State Ion. 2017, 306, 55–61. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kolchugin, A.A. The Influence of the Substituting Element (M = Ca, Sr, Ba) in La1.7M0.3NiO4+δ on the Electrochemical Performance of the Composite Electrodes. Eur. Chem. Technol. J. 2016, 18, 3. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Koroleva, M.; Vdovin, G.; Farlenkov, A.; Medvedev, D. Functionality of an Oxygen Ca3Co4O9+δ Electrode for Reversible Solid Oxide Electrochemical Cells Based on Proton-Conducting Electrolytes. J. Power Sources 2019, 438, 226996. [Google Scholar] [CrossRef]
- Filonova, E.A.; Tokareva, E.S.; Pikalova, N.S.; Vylkov, A.I.; Bogdanovich, N.M.; Pikalova, E.Y. Assessment of Prospective Cathodes Based on (1 − x)Ca3Co4O9+δ–xBaCe0.5Zr0.3Y0.1Yb0.1O3 − δ Composites for Protonic Ceramic Electrochemical Cells. J. Solid State Electrochem. 2020, 24, 1509–1521. [Google Scholar] [CrossRef]
- Chen, K.; Ai, N.; Jiang, S.P. Performance and Structural Stability of Gd0.2Ce0.8O1.9 Infiltrated La0.8Sr0.2MnO3 Nano-Structured Oxygen Electrodes of Solid Oxide Electrolysis Cells. Int. J. Hydrogen Energy 2014, 39, 10349–10358. [Google Scholar] [CrossRef]
- Zha, S.; Zhang, Y.; Liu, M. Functionally Graded Cathodes Fabricated by Sol-Gel/Slurry Coating for Honeycomb SOFCs. Solid State Ion. 2005, 176, 25–31. [Google Scholar] [CrossRef]
- Kammer Hansen, K.; Menon, M.; Knudsen, J.; Bonanos, N.; Mogensen, M. The Effect of a CGO Barrier Layer on the Performance of LSM/YSZ SOFC Cathodes. J. Electrochem. Soc. 2010, 157, B309. [Google Scholar] [CrossRef]
- Recio, P.; Alcázar, C.; Moreno, R. Colloidal Processing of Y0.08Zr0.92O2/La0.80Sr0.20MnO3 Semi-Cells Using a Sr-Doped Lanthanum Manganite Synthesized by a Citrate Route. Materials 2021, 14, 7831. [Google Scholar] [CrossRef]
- Chen, M.; Moon, B.H.; Kim, S.H.; Kim, B.H.; Xu, Q.; Ahn, B.-G. Characterization of La0.6Sr0.4Co0.2Fe0.8O3 − δ + La2NiO4+δ Composite Cathode Materials for Solid Oxide Fuel Cells. Fuel Cells 2012, 12, 86–96. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, F.; Ding, D.; Gao, J. Development and Fabrication of a New Concept Planar-Tubular Solid Oxide Fuel Cell (PT-SOFC). Fuel Cells 2011, 11, 451–458. [Google Scholar] [CrossRef]
- Zunic, M.; Chevallier, L.; Di Bartolomeo, E.; D’Epifanio, A.; Licoccia, S.; Traversa, E. Anode Supported Protonic Solid Oxide Fuel Cells Fabricated Using Electrophoretic Deposition. Fuel Cells 2011, 11, 165–171. [Google Scholar] [CrossRef]
- Harboe, S.; Lupetin, P.; Guillon, O.; Menzler, N.H. Investigation of LSM/8YSZ Cathode within an All-Ceramic SOFC, Part II: Optimization of Performance and Co-Sinterability. J. Eur. Ceram. Soc. 2020, 40, 3618–3631. [Google Scholar] [CrossRef]
- Liang, M.; Yu, B.; Wen, M.; Chen, J.; Xu, J.; Zhai, Y. Preparation of LSM–YSZ Composite Powder for Anode of Solid Oxide Electrolysis Cell and Its Activation Mechanism. J. Power Sources 2009, 190, 341–345. [Google Scholar] [CrossRef]
- Gong, Y.; Ji, W.; Zhang, L.; Xie, B.; Wang, H. Performance of (La,Sr)MnO3 Cathode Based Solid Oxide Fuel Cells: Effect of Bismuth Oxide Sintering Aid in Silver Paste Cathode Current Collector. J. Power Sources 2011, 196, 928–934. [Google Scholar] [CrossRef]
- Tanveer, W.H.; Rezk, H.; Nassef, A.; Abdelkareem, M.A.; Kolosz, B.; Karuppasamy, K.; Aslam, J.; Gilani, S.O. Improving Fuel Cell Performance via Optimal Parameters Identification through Fuzzy Logic Based-Modeling and Optimization. Energy 2020, 204, 117976. [Google Scholar] [CrossRef]
- Pijolat, C. Screen-Printing for the Fabrication of Solid Oxide Fuel Cells (SOFC). In Printed Films; Elsevier: Amsterdam, The Netherlands, 2012; pp. 469–495. ISBN 978-1-84569-988-8. [Google Scholar]
- Park, S.-C.; Lee, J.-J.; Lee, S.-H.; Moon, J.; Hyun, S.-H. Design and Preparation of SOFC Unit Cells Using Scandia-Stabilized Zirconia Electrolyte for Intermediate Temperature Operation. J. Fuel Cell Sci. Technol. 2011, 8, 044501. [Google Scholar] [CrossRef]
- Li, D.; Jin, Y.; Liu, C.; Fu, M.; Zong, X.; Xiong, Y. Effect of Optimal GDC Loading on Long-Term Stability of La0.8Sr0.2Co0.2Fe0.8O3 − δ/Gd0.2Ce0.8O1.9 Composite Cathodes. Ceram. Int. 2021, 47, 6591–6596. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, M.; Ye, F.; Bai, Y.; Li, M.; Fan, S.; Zhang, L. Structure Design, Fabrication, Properties of Laminated Ceramics: A Review. Int. J. Lightweight Mater. Manufact. 2018, 1, 126–141. [Google Scholar] [CrossRef]
- Liu, T.; Lin, J.; Liu, T.; Wu, H.; Xia, C.; Chen, C.; Zhan, Z. Tailoring the Pore Structure of Cathode Supports for Improving the Electrochemical Performance of Solid Oxide Fuel Cells. J. Electroceram. 2018, 40, 138–143. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Sun, Y.; Chen, T.; Zhang, B.; Li, F.; Zhou, J.; Wang, S. Fabrication of Anode Supported Solid Oxide Electrolysis Cell with the Co-Tape Casting Technique and Study on Co-Electrolysis Characteristics. J. Power Sources 2023, 569, 232912. [Google Scholar] [CrossRef]
- Won, B.-R.; Kim, Y.H.; Jo, S.; Myung, J. Highly Flexible Solid Oxide Fuel Cells Using Phase-Controlled Electrolyte Support. J. Eur. Ceram. Soc. 2022, 42, 5813–5819. [Google Scholar] [CrossRef]
- Snowdon, A.L.; Jiang, Z.; Steinberger-Wilckens, R. Five-layer Reverse Tape Casting of IT-SOFC. Int. J. Appl. Ceram. Technol. 2022, 19, 289–298. [Google Scholar] [CrossRef]
- Xu, N.; Geng, D.; Tong, X.; Sun, M.; Xu, Z. Fabrication and Characterization of Co-Fired Metal-Supported Solid Oxide Fuel Cells. Solid State Ion. 2020, 358, 115482. [Google Scholar] [CrossRef]
- Shimada, H.; Fujimaki, Y.; Fujishiro, Y. Highly Active and Durable La0.4Sr0.6MnO3 − δ and Ce0.8Gd0.2O1.9 Nanocomposite Electrode for High-Temperature Reversible Solid Oxide Electrochemical Cells. Ceram. Int. 2020, 46, 19617–19623. [Google Scholar] [CrossRef]
- Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Boosting the Performance of La0.8Sr0.2MnO3 − δ Electrodes by the Incorporation of Nanocomposite Active Layers. Adv. Mater. Int. 2022, 9, 2200702. [Google Scholar] [CrossRef]
- Vardavoulias, M.; Gkomoza, P.; Arkas, M.; Niakolas, D.K.; Neophytides, S.G. Thermal Spray Multilayer Ceramic Structures with Potential for Solid Oxide Cell Applications. Coatings 2021, 11, 682. [Google Scholar] [CrossRef]
- Pyo, S.-S.; Lee, S.-B.; Lim, T.-H.; Song, R.-H.; Shin, D.-R.; Hyun, S.-H.; Yoo, Y.-S. Characteristic of (La0.8Sr0.2)0.98MnO3 Coating on Crofer22APU Used as Metallic Interconnects for Solid Oxide Fuel Cell. Int. J. Hydrogen Energy 2011, 36, 1868–1881. [Google Scholar] [CrossRef]
- Lin, J.; Li, H.; Wang, W.; Qiu, P.; Tao, G.; Huang, K.; Chen, F. Atmospheric Plasma Spraying to Fabricate Metal-Supported Solid Oxide Fuel Cells with Open-Channel Porous Metal Support. J. Am. Ceram. Soc. 2023, 106, 68–78. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; Porras-Vázquez, J.M.; Martín, F.; Ramos-Barrado, J.R.; Losilla, E.R.; Marrero-López, D. A Novel Multilaminated Composite Cathode for Solid Oxide Fuel Cells. Ceram. Int. 2019, 45, 18124–18127. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Zhang, W.; Wei, Z.; Guan, K.; Meng, J.; Meng, F.; Meng, J.; Liu, X. Enhancing Catalysis Activity of La0.6Sr0.4Co0.8Fe0.2O3 − δ Cathode for Solid Oxide Fuel Cell by a Facile and Efficient Impregnation Process. Int. J. Hydrogen Energy 2019, 44, 13757–13767. [Google Scholar] [CrossRef]
- Ahamad, S.; Ahmad, M.; Mehta, B.R.; Gupta, A. Effect of Nano-Fillers on Capacity Retention and Rate Capability of Mesocarbon Microbeads Anode. J. Electrochem. Soc. 2017, 164, A2967–A2976. [Google Scholar] [CrossRef]
- Verma, R.; Suri, N.M.; Kant, S. Effect of Parameters on Adhesion Strength for Slurry Spray Coating Technique. Mater. Manufact. Proc. 2017, 32, 416–424. [Google Scholar] [CrossRef]
- Creanga, C.; Serban, S.; Pittson, R.; Murr, N.E. “No Calibration” Type Sensor in Routine Amperometric Bio-sensing: An Example of a Disposable Hydrogen Peroxide Biosensor. In Biosensors—Emerging Materials and Applications; InTechOpen: London, UK, 2011; pp. 141–152. [Google Scholar] [CrossRef]
- Ren, L.; Luo, X.; Zhou, H. The Tape Casting Process for Manufacturing Low-temperature Co-fired Ceramic Green Sheets: A Review. J. Am. Ceram. Soc. 2018, 101, 3874–3889. [Google Scholar] [CrossRef]
- Emley, B.; Panthi, D.; Du, Y.; Yao, Y. Controlling Porosity of Anode Support in Tubular Solid Oxide Fuel Cells by Freeze Casting. J. Electrochem. Energy Convers. Storage 2020, 17, 041008. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Ma, Y.; Ye, H.; Liu, Y.; Liu, J.; Zhao, X.; Tao, T.; Yao, Y.; Lu, S.; et al. Preparation and Performance of a Nano-Honeycomb Cathode for Microtubular Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2023, 48, 5229–5236. [Google Scholar] [CrossRef]
- Sahu, S.K.; Panthi, D.; Feng, H.; Du, Y. Effect of Microstructure of the Anode Support Tubes towards SOFC Performance. In Proceedings of the ECS Transactions; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 103, pp. 407–418. [Google Scholar]
- Timurkutluk, C.; Yildirim, F.; Toruntay, F.; Onbilgin, S.; Yagiz, M.; Timurkutluk, B. Fabrication and Optimization of LSM Infiltrated Cathode Electrode for Anode Supported Microtubular Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2023, 48, 9833–9844. [Google Scholar] [CrossRef]
- Panthi, D.; Choi, B.; Tsutsumi, A. A Novel Micro-Tubular Solid Oxide Fuel Cell with a Porous Zirconia Support for Intermediate-Temperature Operation. ECS Trans. 2015, 68, 2259–2265. [Google Scholar] [CrossRef]
- Min, S.H.; Song, R.-H.; Lee, J.G.; Park, M.-G.; Ryu, K.H.; Jeon, Y.-K.; Shul, Y. Fabrication of Anode-Supported Tubular Ba(Zr0.1Ce0.7Y0.2)O3 − δ Cell for Intermediate Temperature Solid Oxide Fuel Cells. Ceram. Int. 2014, 40, 1513–1518. [Google Scholar] [CrossRef]
- Zhao, K.; Kim, B.-H.; Norton, M.G.; Ha, S.Y. Cathode Optimization for an Inert-Substrate-Supported Tubular Solid Oxide Fuel Cell. Front. Energy Res. 2018, 6, 87. [Google Scholar] [CrossRef]
- Amiri, T.; Singh, K.; Sandhu, N.K.; Hanifi, A.R.; Etsell, T.H.; Luo, J.-L.; Thangadurai, V.; Sarkar, P. High Performance Tubular Solid Oxide Fuel Cell Based on Ba0.5Sr0.5Ce0.6Zr0.2Gd0.1Y0.1O3 − δ Proton Conducting Electrolyte. J. Electrochem. Soc. 2018, 165, F764–F769. [Google Scholar] [CrossRef]
- López-Robledo, M.J.; Laguna-Bercero, M.A.; Silva, J.; Orera, V.M.; Larrea, A. Electrochemical Performance of Intermediate Temperature Micro-Tubular Solid Oxide Fuel Cells Using Porous Ceria Barrier Layers. Ceram. Int. 2015, 41, 7651–7660. [Google Scholar] [CrossRef]
- Mushtaq, U.; Kim, D.-W.; Yun, U.-J.; Lee, J.-W.; Lee, S.-B.; Park, S.-J.; Song, R.-H.; Kim, G.; Lim, T.-H. Effect of Cathode Geometry on the Electrochemical Performance of Flat Tubular Segmented-in-Series (SIS) Solid Oxide Fuel Cell. Int. J. Hydrogen Energy 2015, 40, 6207–6215. [Google Scholar] [CrossRef]
- Ab Rahman, M.; Othman, M.H.D.; Fansuri, H.; Harun, Z.; Omar, A.F.; Shabri, H.A.; Ravi, J.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; et al. Development of High-Performance Anode/Electrolyte/Cathode Micro-Tubular Solid Oxide Fuel Cell via Phase Inversion-Based Co-Extrusion/Co-Sintering Technique. J. Power Sources 2020, 467, 228345. [Google Scholar] [CrossRef]
- ur Rehman, S.; Song, R.-H.; Lee, J.-W.; Lim, T.-H.; Park, S.-J.; Lee, S.-B. Fabrication and Characterization of La0.65Sr0.3MnO3 − δ/(Y2O3)0.08(ZrO2)0.92/Gd0.1Ce0.9O2 − δ Tri-Composite Cathode-Supported Tubular Direct Carbon Solid Oxide Fuel Cell. Ceram. Int. 2017, 43, 1086–1091. [Google Scholar] [CrossRef]
- Ab Rahman, M.; Othman, M.H.D.; Fansuri, H.; Harun, Z.; Jamil, S.M.; Omar, A.F.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Effect of Sintering Temperature on Perovskite-Based Hollow Fiber as a Substrate for Cathode-Supported Micro-Tubular Solid Oxide Fuel Cell. J. Aust. Ceram. Soc. 2021, 57, 1199–1208. [Google Scholar] [CrossRef]
- Wang, S.-F.; Hsu, Y.-F.; Hsia, P.; Hung, W.-K.; Jasinski, P. Design and Characterization of Apatite La9.8Si5.7Mg0.3O26±δ-Based Micro-Tubular Solid Oxide Fuel Cells. J. Power Sources 2020, 460, 228072. [Google Scholar] [CrossRef]
- Jamil, S.M.; Dzarfan Othman, M.H.; Mohamed, M.H.; Adam, M.R.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. A Novel Single-Step Fabrication Anode/Electrolyte/Cathode Triple-Layer Hollow Fiber Micro-Tubular SOFC. Int. J. Hydrogen Energy 2018, 43, 18509–18515. [Google Scholar] [CrossRef]
- López-Robledo, M.J.; Laguna-Bercero, M.A.; Larrea, A.; Orera, V.M. Reversible Operation of Microtubular Solid Oxide Cells Using La0.6Sr0.4Co0.2Fe0.8O3 − δ−Ce0.9Gd0.1O2 − δ Oxygen Electrodes. J. Power Sources 2018, 378, 184–189. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Place of Electrophoretic Deposition among Thin-Film Methods Adapted to the Solid Oxide Fuel Cell Technology: A Short Review. Int. J. Energy Prod. Manag. 2019, 4, 1–27. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y. New Trends in the Development of Electrophoretic Deposition Method in the Solid Oxide Fuel Cell Technology: Theoretical Approaches, Experimental Solutions and Development Prospects. Russ. Chem. Rev. 2019, 88, 1179–1219. [Google Scholar] [CrossRef]
- Yamaji, T.; Itagaki, Y.; Arakawa, K.; Sadaoka, Y. Formation of La0.8Sr0.2MnO3 Films as SOFC Cathodes by Electrophoretic Deposition. J. Ceram. Soc. Jpn. 2010, 118, 1202–1206. [Google Scholar] [CrossRef]
- Cherng, J.S.; Wu, C.C.; Chen, W.H.; Yeh, T.H. Microstructure and Performance of Micro-Tubular Solid Oxide Fuel Cells Made by Aqueous Electrophoretic Deposition. Ceram. Int. 2013, 39, S601–S604. [Google Scholar] [CrossRef]
- Cherng, J.S.; Ho, M.Y.; Yeh, T.H.; Chen, W.H. Anode-Supported Micro-Tubular SOFCs Made by Aqueous Electrophoretic Deposition. Ceram. Int. 2012, 38, S477–S480. [Google Scholar] [CrossRef]
- Salehzadeh, D.; Torabi, M.; Sadeghian, Z.; Marashi, P. A Multiscale-Architecture Solid Oxide Fuel Cell Fabricated by Electrophoretic Deposition Technique. J. Alloys Compd. 2020, 830, 154654. [Google Scholar] [CrossRef]
- Santillán, M.J.; Caneiro, A.; Quaranta, N.; Boccaccini, A.R. Electrophoretic Deposition of La0.6Sr0.4Co0.8Fe0.2O3 − δ Cathodes on Ce0.9Gd0.1O1.95 Substrates for Intermediate Temperature Solid Oxide Fuel Cell (IT-SOFC). J. Eur. Ceram. Soc. 2009, 29, 1125–1132. [Google Scholar] [CrossRef]
- Santillán, M.J.; Caneiro, A.; Lovey, F.C.; Quaranta, N.; Boccaccini, A.R. Electrophoretic Codeposition of La0.6Sr0.4Co0.8Fe0.2O3 − δ and Carbon Nanotubes for Developing Composite Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. Int. J. Appl. Ceram. Technol. 2010, 7, 30–40. [Google Scholar] [CrossRef]
- Baharuddin, N.A. Influence of Sintering Temperature on the Polarization Resistance of La0.6Sr0.4Co0.2Fe0.8O3 − δ−SDC Carbonate Composite Cathode. Ceram. Silikáty 2016, 60, 115–121. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sato, K.; Matsuda, M.; Ozawa, M.; Ohara, S. Anomalous Low-Temperature Sintering of a Solid Electrolyte Thin Film of Tailor-Made Nanocrystals on a Porous Cathode Support for Low-Temperature Solid Oxide Fuel Cells. Ceram. Int. 2021, 47, 15939–15946. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Chang, Y.-T.; Wu, P.-W.; Lin, P. Fabrication of Tri-Layered Structrure for Solid Oxide Fuel Cells by Electrophetic Depositions. ECS Trans. 2009, 25, 643–648. [Google Scholar] [CrossRef]
- Itagaki, Y.; Watanabe, S.; Yamaji, T.; Asamoto, M.; Yahiro, H.; Sadaoka, Y. Electrophoretic Deposition of Bi-Layered LSM/LSM–YSZ Cathodes for Solid Oxide Fuel Cell. J. Power Sources 2012, 214, 153–158. [Google Scholar] [CrossRef]
- Ji, Q.; Xu, X.; Liu, X.; Bi, L. Improvement of the Catalytic Properties of Porous Lanthanum Manganite for the Oxygen Reduction Reaction by Partial Substitution of Strontium for Lanthanum. Electrochem. Commun. 2021, 124, 106964. [Google Scholar] [CrossRef]
- Torabi, A.; Hanifi, A.R.; Etsell, T.H.; Sarkar, P. Effects of Porous Support Microstructure on Performance of Infiltrated Electrodes in Solid Oxide Fuel Cells. J. Electrochem. Soc. 2011, 159, B201–B210. [Google Scholar] [CrossRef]
- Benipayo, A.B.; Cervera, R.B.M. Influence of Carbon Black Pore Former on the Synthesis of LSM-YSZ Composite Electrode Material via Solid-State Reaction and Glycine-Nitrate Process. Mater. Sci. Forum 2019, 950, 154–159. [Google Scholar] [CrossRef]
- Noh, J.H.; Myung, J. Optimization of Anode and Electrolyte Microstructure for Solid Oxide Fuel Cells. Korean Chem. Eng. Res. 2019, 57, 525–530. [Google Scholar] [CrossRef]
- Wu, S.; Tan, W.; Peng, Y.; Gao, J.; Shi, H.; Qu, J.; Zhu, X. Electrochemical Reduction of Nitric Oxide in Different Carbon-Driven Solid State Cells. J. Alloys Compd. 2020, 812, 152163. [Google Scholar] [CrossRef]
- Nie, L.; Liu, J.; Zhang, Y.; Liu, M. Effects of Pore Formers on Microstructure and Performance of Cathode Membranes for Solid Oxide Fuel Cells. J. Power Sources 2011, 196, 9975–9979. [Google Scholar] [CrossRef]
- Mohamed, R.; Cheng, X.; Fabbri, E.; Levecque, P.; Kötz, R.; Conrad, O.; Schmidt, T.J. Electrocatalysis of Perovskites: The Influence of Carbon on the Oxygen Evolution Activity. J. Electrochem. Soc. 2015, 162, F579–F586. [Google Scholar] [CrossRef]
- Safakas, A.; Bampos, G.; Bebelis, S. Oxygen Reduction Reaction on La0.8Sr0.2CoxFe1 − xO3 − δ Perovskite/Carbon Black Electrocatalysts in Alkaline Medium. Appl. Catal. B Environ. 2019, 244, 225–232. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X. A Platinum Nanowire Network as a Highly Efficient Current Collector for Intermediate Temperature Solid Oxide Fuel Cells. RSC Adv. 2014, 4, 11317–11321. [Google Scholar] [CrossRef]
- Burmistrov, I.N.; Agarkov, D.A.; Tsybrov, F.M.; Bredikhin, S.I. Preparation of Membrane-Electrode Assemblies of Solid Oxide Fuel Cells by Co-Sintering of Electrodes. Russ. J. Electrochem. 2016, 52, 669–677. [Google Scholar] [CrossRef]
- Burmistrov, I.N.; Agarkov, D.A.; Korovkin, E.V.; Yalovenko, D.V.; Bredikhin, S.I. Fabrication of Membrane–Electrode Assemblies for Solid-Oxide Fuel Cells by Joint Sintering of Electrodes at High Temperature. Russ. J. Electrochem. 2017, 53, 873–879. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, G.; Zeng, S.; Parbey, J.; Xiao, S.; Li, B.; Li, T.; Andersson, M. Mechanism of Chromium Poisoning the Conventional Cathode Material for Solid Oxide Fuel Cells. J. Power Sources 2018, 381, 26–29. [Google Scholar] [CrossRef]
- He, Z.; Andersen, K.B.; Keel, L.; Nygaard, F.B.; Menon, M.; Hansen, K.K. Processing and Characterization of Porous Electrochemical Cells for Flue Gas Purification. Ionics 2009, 15, 427–431. [Google Scholar] [CrossRef]
- Abd Mutalib, M.; Othman, M.H.D.; Aziz, M.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; Mohamed, M.A. The Influence of PEEK as a Pore Former on the Microstructure of Brush-Painted LSCF Cathodes. J. Solid State Electrochem. 2016, 20, 2895–2905. [Google Scholar] [CrossRef]
- Duan, N.-Q.; Tan, Y.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. Biomass Carbon Fueled Tubular Solid Oxide Fuel Cells with Molten Antimony Anode. Appl. Energy 2016, 165, 983–989. [Google Scholar] [CrossRef]
- Chen, M.; Kim, B.H.; Xu, Q.; Ahn, B.G.; Huang, D.P. Fabrication and Performance of Anode-Supported Solid Oxide Fuel Cells via Slurry Spin Coating. J. Membr. Sci. 2010, 360, 461–468. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Recent Progress in Nanostructured Electrodes for Solid Oxide Fuel Cells Deposited by Spray Pyrolysis. J. Power Sources 2021, 507, 230277. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Highly Efficient La0.8Sr0.2MnO3 − δ−Ce0.9Gd0.1O1.95 Nanocomposite Cathodes for Solid Oxide Fuel Cells. Ceram. Int. 2018, 44, 4961–4966. [Google Scholar] [CrossRef]
- Kumari, N.; Haider, M.A.; Anjum, U.; Basu, S. Identifying Operating Mechanism in the Electrochemical Reduction of CO2 on Thin-Film Praseodymium-Doped Ceria Electrodes. Ionics 2020, 26, 5673–5684. [Google Scholar] [CrossRef]
- Krestou, A.; Barmpatsis, A.; Tsanaktsidis, C.; Matsouka, C.; Nalbandian, L.; Kiratzis, N.E. Fabrication and Characterization of Functional Ceramic Films by Solution Spray Pyrolysis: Correlations with the Thermal Decomposition Characteristics of the Constituent Salts. ECS Trans. 2021, 103, 123–138. [Google Scholar] [CrossRef]
- Sharma, R.K.; Khamidy, N.I.; Rapenne, L.; Charlot, F.; Moussaoui, H.; Laurencin, J.; Djurado, E. Highly Efficient Architectured Pr6O11 Oxygen Electrode for Solid Oxide Fuel Cell. J. Power Sources 2019, 419, 171–180. [Google Scholar] [CrossRef]
- Castro-Robles, J.D.; Soltani, N.; Chávez-Carvayar, J.Á. Structural, Morphological and Transport Properties of Nanostructured La1 − xSrxCo0.2Fe0.8O3 − δ Thin Films, Deposited by Ultrasonic Spray Pyrolysis. Mater. Chem. Phys. 2019, 225, 50–54. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Highly Oriented and Fully Dense CGO Films Prepared by Spray-Pyrolysis and Different Precursor Salts. J. Eur. Ceram. Soc. 2020, 40, 3080–3088. [Google Scholar] [CrossRef]
- Kamecki, B.; Karczewski, J.; Jasiński, P.; Molin, S. Improvement of Oxygen Electrode Performance of Intermediate Temperature Solid Oxide Cells by Spray Pyrolysis Deposited Active Layers. Adv. Mater. Interfaces 2021, 8, 2002227. [Google Scholar] [CrossRef]
- Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Unraveling the Influence of the Electrolyte on the Polarization Resistance of Nanostructured La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes. Nanomaterials 2022, 12, 3936. [Google Scholar] [CrossRef]
- Aruna, S.T.; Balaji, L.S.; Kumar, S.S.; Prakash, B.S. Electrospinning in Solid Oxide Fuel Cells—A Review. Renew. Sustain. Energy Rev. 2017, 67, 673–682. [Google Scholar] [CrossRef]
- Parbey, J.; Wang, Q.; Yu, G.; Zhang, X.; Li, T.; Andersson, M. Progress in the Use of Electrospun Nanofiber Electrodes for Solid Oxide Fuel Cells: A Review. Rev. Chem. Eng. 2020, 36, 879–931. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, Y.; Bi, L. Applications of Electrospun Nanofibers in Solid Oxide Fuel Cells—A Review. J. Alloys Compd. 2023, 937, 168288. [Google Scholar] [CrossRef]
- Squizzato, E.; Sanna, C.; Glisenti, A.; Costamagna, P. Structural and Catalytic Characterization of La0.6Sr0.4MnO3 Nanofibers for Application in Direct Methane Intermediate Temperature Solid Oxide Fuel Cell Anodes. Energies 2021, 14, 3602. [Google Scholar] [CrossRef]
- Gong, J.; Wu, P.; Bai, Z.; Ma, J.; Li, T.; Yao, Y.; Jiang, C. Insight into the Electrospinning Process for SOFC Cathode Nanofibers. J. Phys. Chem. C 2021, 125, 7044–7053. [Google Scholar] [CrossRef]
- Enrico, A.; Zhang, W.; Lund Traulsen, M.; Sala, E.M.; Costamagna, P.; Holtappels, P. La0.6Sr0.4Co0.2Fe0.8O3 − δ Nanofiber Cathode for Intermediate-Temperature Solid Oxide Fuel Cells by Water-Based Sol-Gel Electrospinning: Synthesis and Electrochemical Behaviour. J. Eur. Ceram. Soc. 2018, 38, 2677–2686. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.J.; Su, D.L.; Dai, C.S.; Xiong, Y.P. Effect of Microstructure on Electrochemical Performance of Nano-Structured La0.8Sr0.2Co0.2Fe0.8O3 − δ−Gd0.2Ce0.8O1.9 Composite Cathodes. In Proceedings of the ECS Transactions; Eguchi, K., Singhal, S.C., Eds.; Electrochemical Society Inc.: Philadelphia, PA, USA, 2019; Volume 91, pp. 1483–1489. [Google Scholar]
- Zhang, W.; Wang, H.; Guan, K.; Wei, Z.; Zhang, X.; Meng, J.; Liu, X.; Meng, J. La0.6Sr0.4Co0.2Fe0.8O3 − δ/CeO2 Heterostructured Composite Nanofibers as a Highly Active and Robust Cathode Catalyst for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 26830–26841. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Zhang, W.; Costamagna, P.; Holtappels, P. Synthesis and Electrochemical Characterization of La0.6Sr0.4Co0.2Fe0.8O3 − δ/Ce0.9Gd0.1O1.95 Co-Electrospun Nanofiber Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 13818–13831. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, D.; Zhu, X.; Wang, N.; Chen, R.; Wang, B. New SOFC Cathode: 3D Core–Shell-Structured La0.6Sr0.4Co0.2Fe0.8O3 − δ@PrO2 − δ Nanofibers Prepared by Coaxial Electrospinning. ACS Appl. Energy Mater. 2022, 5, 11178–11190. [Google Scholar] [CrossRef]
- Chen, S.-H.; Zhang, T.-T.; Zhu, D.-Y.; Wang, N.; Xu, S.; Ramakrishna, S.; Long, Y.-Z. Synthesis and Electrochemical Characterization of La0.6Sr0.4Co0.2Fe0.8O3 − δ and BaZr0.8Y0.2O3 − δ Electrospun Nanofiber Cathodes for Solid Oxide Fuel Cells. Adv. Eng. Mater. 2022, 24, 2101083. [Google Scholar] [CrossRef]
- Hedayat, N.; Du, Y. Micro-Tubular Solid Oxide Fuel Cells with One Closed-End Fabricated via Dip-Coating and Co-Firing. ECS Trans. 2021, 103, 113–121. [Google Scholar] [CrossRef]
- Anelli, S.; Baiutti, F.; Hornés, A.; Bernadet, L.; Torrell, M.; Tarancón, A. Improved Mesostructured Oxygen Electrodes for Highly Performing Solid Oxide Cells for Co-Electrolysis of Steam and Carbon Dioxide. J. Mater. Chem. A 2019, 7, 27458–27468. [Google Scholar] [CrossRef]
- Yan, Z.; He, A.; Hara, S.; Shikazono, N. In-Silico Design of Functionally Graded Electrodes for Solid Oxide Fuel Cells. In Proceedings of the ECS Transactions; Eguchi, K., Singhal, S.C., Eds.; Electrochemical Society Inc.: Philadelphia, PA, USA, 2019; Volume 91, pp. 2055–2064. [Google Scholar]
- Muhoza, S.P.; Gross, M.D. Creating and Preserving Nanoparticles during Co-Sintering of Solid Oxide Electrodes and Its Impact on Electrocatalytic Activity. Catalysts 2021, 11, 1073. [Google Scholar] [CrossRef]
- Muhoza, S.P.; Taylor, T.H.; Song, X.; Gross, M.D. The Impact of Sintering Atmosphere and Temperature on the Phase Evolution of High Surface Area LSCF Prepared by in Situ Carbon Templating. J. Electrochem. Soc. 2021, 168, 034519. [Google Scholar] [CrossRef]
- Hedayat, N.; Du, Y.; Ilkhani, H. Review on Fabrication Techniques for Porous Electrodes of Solid Oxide Fuel Cells by Sacrificial Template Methods. Renew. Sustain. Energy Rev. 2017, 77, 1221–1239. [Google Scholar] [CrossRef]
- Kim, S.; Kim, G.; Manthiram, A. A Review on Infiltration Techniques for Energy Conversion and Storage Devices: From Fundamentals to Applications. Sustain. Energy Fuels 2021, 5, 5024–5037. [Google Scholar] [CrossRef]
- Vohs, J.M.; Gorte, R.J. High-Performance SOFC Cathodes Prepared by Infiltration. Adv. Mater. 2009, 21, 943–956. [Google Scholar] [CrossRef]
- Biswas, S.; Kaur, G.; Paul, G.; Giddey, S. A Critical Review on Cathode Materials for Steam Electrolysis in Solid Oxide Electrolysis. Int. J. Hydrogen Energy 2023, 48, 12541–12570. [Google Scholar] [CrossRef]
- He, S.; Zou, Y.; Chen, K.; Li, N.; Li, D.; Jiang, S.P. A Critical Review of the Nano-Structured Electrodes of Solid Oxide Cells. Chem. Commun. 2022, 58, 10619–10626. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, L.; Paredes Navia, S.A.; Hinerman, A.; Gerdes, K.; Song, X. Synergetic Interaction of Additive Dual Nanocatalysts to Accelerate Oxygen Reduction Reaction in Fuel Cell Cathodes. ACS Catal. 2019, 9, 6664–6671. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Wang, H.; Yang, T.; Lu, M.Y.; Barnett, S.A. Characteristics of Oxygen Electrode Supported Reversible Solid Oxide Cells. J. Electrochem. Soc. 2021, 168, 054504. [Google Scholar] [CrossRef]
- Han, F.-Z.; Zhang, J.-H.; Zhang, S.-L.; Li, C.-X. Comparative Study on the Electrochemical Performance of Sr(Ti0.3Fe0.7)O3 − δ Fuel Electrode in Different Fuel Gases for Solid Oxide Electrochemical Cells. Ceram. Int. 2023, 49, 2410–2418. [Google Scholar] [CrossRef]
- Orera, A.; Betato, A.; Silva-Treviño, J.; Larrea, Á.; Laguna-Bercero, M.Á. Advanced Metal Oxide Infiltrated Electrodes for Boosting the Performance of Solid Oxide Cells. J. Mater. Chem. A 2022, 10, 2541–2549. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, P.; Zhang, X.; Ding, L.; Li, Y.; Wu, X. Characterization of La0.65Sr0.35MnO3-Based Double-Layered Oxygen Electrode for Reversible Solid Oxide Cells. Ionics 2022, 28, 801–812. [Google Scholar] [CrossRef]
- Koo, J.Y.; Mun, T.; Lee, J.; Choi, M.; Kim, S.J.; Lee, W. Enhancement of Oxygen Reduction Reaction Kinetics Using Infiltrated Yttria-Stabilized Zirconia Interlayers at the Electrolyte/Electrode Interfaces of Solid Oxide Fuel Cells. J. Power Sources 2020, 472, 228606. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, F.; Yan, M.; Wan, Y.; Jiao, Z.; Xia, C.; Chen, F.; Ni, M. High-Throughput, Super-Resolution 3D Reconstruction of Nano-Structured Solid Oxide Fuel Cell Electrodes and Quantification of Microstructure-Property Relationships. J. Power Sources 2019, 427, 112–119. [Google Scholar] [CrossRef]
- Wan, S.; Yan, M.; Zhang, Y. A Numerical Study of Infiltrated Solid Oxide Fuel Cell Electrode with Dual-phase Backbone. Int. J. Energy Res. 2019, 43, 2562–2570. [Google Scholar] [CrossRef]
- Kim, S.J.; Koo, J.Y.; Mun, T.; Choi, M.; Lee, W. Tailoring Defect Chemistry at Interfaces for Promoted Oxygen Reduction Reaction Kinetics. J. Mater. Chem. A 2020, 8, 23313–23322. [Google Scholar] [CrossRef]
- Yang, T.; Kollasch, S.L.; Grimes, J.; Xue, A.; Barnett, S.A. (La0.8Sr0.2)0.98MnO3 − δ–Zr0.92Y0.16O2 − δ:PrOx for Oxygen Electrode Supported Solid Oxide Cells. Appl. Catal. B Environ. 2022, 306, 121114. [Google Scholar] [CrossRef]
- Lu, M.Y.; Scipioni, R.; Park, B.-K.; Yang, T.; Chart, Y.A.; Barnett, S.A. Mechanisms of PrOx Performance Enhancement of Oxygen Electrodes for Low and Intermediate Temperature Solid Oxide Fuel Cells. Mater. Today Energy 2019, 14, 100362. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, Y.; Li, M.; Ding, X. In-Situ Strategy to Suppress Chromium Poisoning on La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes of Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2019, 44, 30401–30408. [Google Scholar] [CrossRef]
- Soltanizade, A.; Babaei, A.; Ataie, A.; Seyed-Vakili, S.V. Temperature Dependency of Activity of Nano-Catalysts on La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathode of Solid Oxide Fuel Cells. J. Appl. Electrochem. 2019, 49, 1113–1122. [Google Scholar] [CrossRef]
- Sındıraç, C.; Büyükaksoy, A.; Akkurt, S. Electrochemical Performance of La0.6Sr0.4Co0.2Fe0.8O3–Ce0.9Gd0.1O2 − δ Composite SOFC Cathodes Fabricated by Electrocatalyst and/or Electrocatalyst-Ionic Conductor Infiltration. J. Sol Gel Sci. Technol. 2019, 92, 45–56. [Google Scholar] [CrossRef]
- Huang, Z.; Qi, H.; Zhao, Z.; Shang, L.; Tu, B.; Cheng, M. Efficient CO2 Electroreduction on a Solid Oxide Electrolysis Cell with La0.6Sr0.4Co0.2Fe0.8O3 − δ–Gd0.2Ce0.8O2 − δ Infiltrated Electrode. J. Power Sources 2019, 434, 226730. [Google Scholar] [CrossRef]
- Ge, L.; Sun, K.; Gu, Y.; Ni, Q.; Huang, X. Boosting the Performance of Conventional Air Electrodes for Solid Oxide Cells by In-Situ Loading of Nano Praseodymium Oxide. Energy Convers. Manag. 2021, 249, 114873. [Google Scholar] [CrossRef]
- Brito, M.E.; Morishita, H.; Yamada, J.; Nishino, H.; Uchida, H. Further Improvement in Performances of La0.6Sr0.4Co0.2Fe0.8O3 − δ—Doped Ceria Composite Oxygen Electrodes with Infiltrated Doped Ceria Nanoparticles for Reversible Solid Oxide Cells. J. Power Sources 2019, 427, 293–298. [Google Scholar] [CrossRef]
- Chen, X.; Ni, W.; Du, X.; Sun, Z.; Zhu, T.; Zhong, Q.; Han, M. Electrochemical Property of Multi-Layer Anode Supported Solid Oxide Fuel Cell Fabricated through Sequential Tape-Casting and Co-Firing. J. Mater. Sci. Technol. 2019, 35, 695–701. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, N.; Fan, H.; Han, M. La0.6Sr0.4Co0.2Fe0.8O3 − δ Nanoparticles Modified Ni-Based Anode for Direct Methane-Fueled SOFCs. In Proceedings of the Energy Procedia; Li, H., Yan, J., Yang, H.-X., Chen, X., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 158, pp. 2250–2255. [Google Scholar]
- Fatah, A.F.B.M.A.; Murat, M.N.; Hamid, N.A. Lscf-Cuo as Promising Cathode for It Sofc. J. Eng. Technol. Sci. 2021, 53, 210411. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.; Li, J.; Guo, X.; Hu, Q.; Yang, Z.; Yu, F.; Li, G. Modified La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes with the Infiltration of Er0.4Bi1.6O3 for Intermediate-Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 22932–22941. [Google Scholar] [CrossRef]
- Zhang, Y.; Nicholas, J.D. Evidence That Surface-Segregated Sr Phases Can Be Removed in LSCF via Ceria Pre-Infiltration, Are Less Apt to Form in SSC. J. Electrochem. Soc. 2021, 168, 024522. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, Y.; Bouwmeester, H.J.M.; Xia, C. PbO Effect on the Oxygen Reduction Reaction in Intermediate-Temperature Solid Oxide Fuel Cell. Int. J. Hydrogen Energy 2020, 45, 25299–25306. [Google Scholar] [CrossRef]
- Qiu, P.; Yang, X.; Zou, L.; Zhu, T.; Yuan, Z.; Jia, L.; Li, J.; Chen, F. LaCrO3-Coated La0.6Sr0.4Co0.2Fe0.8O3 − δ Core-Shell Structured Cathode with Enhanced Cr Tolerance for Intermediateerature Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 29133–29142. [Google Scholar] [CrossRef]
- Park, B.-K.; Barnett, S.A. Boosting Solid Oxide Fuel Cell Performance: Via Electrolyte Thickness Reduction and Cathode Infiltration. J. Mater. Chem. A 2020, 8, 11626–11631. [Google Scholar] [CrossRef]
- Namgung, Y.; Hong, J.; Kumar, A.; Lim, D.-K.; Song, S.-J. One Step Infiltration Induced Multi-Cation Oxide Nanocatalyst for Load Proof SOFC Application. Appl. Catal. B Environ. 2020, 267, 118374. [Google Scholar] [CrossRef]
- Song, Y.-H.; Rehman, S.U.; Kim, H.-S.; Song, H.-S.; Song, R.-H.; Lim, T.-H.; Hong, J.-E.; Park, S.-J.; Huh, J.-Y.; Lee, S.-B. Facile Surface Modification of LSCF/GDC Cathodes by Epitaxial Deposition of Sm0.5Sr0.5CoO3 via Ultrasonic Spray Infiltration. J. Mater. Chem. A 2020, 8, 3967–3977. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, M.; Sun, Z. High Performance and Stability of Nanocomposite Oxygen Electrode for Solid Oxide Cells. Int. J. Hydrogen Energy 2020, 45, 5554–5564. [Google Scholar] [CrossRef]
- Akbari, Z.; Babaei, A. Electrochemical Performance of La0.8Sr0.2MnO3 Oxygen Electrode Promoted by Ruddlesden-Popper Structured La2NiO4. J. Am. Ceram. Soc. 2020, 103, 1332–1342. [Google Scholar] [CrossRef]
- Khoshkalam, M.; Faghihi-Sani, M.A.; Tong, X.; Chen, M.; Hendriksen, P.V. Enhanced Activity of Pr6O11 and CuO Infiltrated Ce0.9Gd0.1O2 Based Composite Oxygen Electrodes. J. Electrochem. Soc. 2020, 167, 024505. [Google Scholar] [CrossRef]
- Fatah, A.F.; Mohamad, A.A.; Muchtar, A.; Hamid, N.A. Physical Characterization of LSCF–CuO via Enhanced Modified Sol-Gel Method for Intermediate Temperature Solid Oxide Fuel Cells (IT-SOFCs). Mater. Today Proc. 2020, 46, 2052–2057. [Google Scholar] [CrossRef]
- Rosli, A.Z.; Somalu, M.R.; Osman, N.; Hamid, N.A. Physical Characterization of LSCF–NiO as Cathode Material for Intermediate Temperature Solid Oxide Fuel Cell (IT-SOFCs). Mater. Today Proc. 2020, 46, 1895–1900. [Google Scholar] [CrossRef]
- Ghamarinia, M.; Babaei, A.; Zamani, C. Electrochemical Characterization of La2NiO4-Infiltrated La0.6Sr0.4Co0.2Fe0.8O3 − δ by Analysis of Distribution of Relaxation Times. Electrochim. Acta 2020, 353, 136520. [Google Scholar] [CrossRef]
- Lu, M.Y.; Yang, T.; Scipioni, R.; Chart, Y.A.; Furlong, A.; Barnett, S.A. Sm0.5Sr0.5CoO3 − δ Surface Modification of La0.6Sr0.4Co0.2Fe0.8O3 − δ−Ce0.9Gd0.1O2 − δ Composite Oxygen Electrodes for Solid Oxide Electrochemical Cells. J. Electrochem. Soc. 2020, 167, 164504. [Google Scholar] [CrossRef]
- Kim, M.; Kim, D.H.; Han, G.D.; Choi, H.J.; Choi, H.R.; Shim, J.H. Lanthanum Strontium Cobaltite-Infiltrated Lanthanum Strontium Cobalt Ferrite Cathodes Fabricated by Inkjet Printing for High-Performance Solid Oxide Fuel Cells. J. Alloys Compd. 2020, 843, 155806. [Google Scholar] [CrossRef]
- Hong, J.; Bhardwaj, A.; Namgung, Y.; Bae, H.; Song, S.-J. Evaluation of the Effects of Nanocatalyst Infiltration on the SOFC Performance and Electrode Reaction Kinetics Using the Transmission Line Model. J. Mater. Chem. A 2020, 8, 23473–23487. [Google Scholar] [CrossRef]
- Rehman, S.U.; Song, H.-S.; Kim, H.-S.; Hassan, M.H.; Joh, D.-W.; Song, R.-H.; Lim, T.-H.; Hong, J.-E.; Park, S.-J.; Lee, S.-B. A Dynamic Infiltration Technique to Synthesize Nanolayered Cathodes for High Performance and Robust Solid Oxide Fuel Cells. J. Energy Chem. 2022, 70, 201–210. [Google Scholar] [CrossRef]
- Zhao, H.; Li, W.; Wang, H.; Zhou, J.; Sun, X.; Wang, E.; Zhao, L.; Dong, B.; Wang, S. Surface Modification of La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathode by Infiltrating A-Site Deficient Non-Strontium La0.94Ni0.6Fe0.4O3 Perovskite for Solid Oxide Fuel Cells. Appl. Surf. Sci. 2022, 572, 151382. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, Y.; Huang, K.; Xiong, X.; Wang, J.; Liu, M.; Ding, D.; Chen, Y.; Liu, T. Surface Enhanced Performance of La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes by Infiltration Pr-Ni-Mn-O Progress. J. Alloys Compd. 2022, 902, 163337. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, X.; Long, C.; Li, Y.; Tao, Z. Performance Optimization for Proton Conducting SOFCs by Impregnating Triple-Conducting NBSCF to Form Composite Cathode. Mater. Lett. 2022, 328, 133181. [Google Scholar] [CrossRef]
- Jeon, S.; Seo, J.; Shin, J.W.; Lee, S.; Seo, H.G.; Lee, S.; Tsvetkov, N.; Kim, J.; An, J.; Jung, W. Metal-Oxide Nanocomposite Catalyst Simultaneously Boosts the Oxygen Reduction Reactivity and Chemical Stability of Solid Oxide Fuel Cell Cathode. Chem. Eng. J. 2023, 455, 140611. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Q.; Jiang, S.P.; Zhao, L.; Ai, N.; Wang, X.; Shao, Y.; Guan, C.; Fang, H.; Luo, Y.; et al. Promotional Role of BaCO3 on the Chromium–Tolerance of La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes of Solid Oxide Fuel Cells. Appl. Catal. B Environ. 2023, 321, 122080. [Google Scholar] [CrossRef]
- Tarragó, D.P.; Moreno, B.; Chinarro, E.; de Fraga Malfatti, C.; de Sousa, V.C. Deposition of Nanostructured LSM Perovskite Thin Film on Dense YSZ Substrate by Airbrushed Solution Combustion (ASC) for Application in SOFC Cathodes. Int. J. Hydrogen Energy 2020, 45, 11749–11760. [Google Scholar] [CrossRef]
- Kevin, B.; Abernathy, H.; Liu, M. Surface Enhanced Raman Spectroscopy for Investigation of SOFC Cathodes. In Proceedings of the Advances in Solid Oxide Fuel Cells V—33rd International Conference on Advanced Ceramics and Composites, Atlanta, GA, USA, 18–23 January 2009; Volume 30, pp. 63–73. [Google Scholar]
- Yoo, Y.-S.; Namgung, Y.; Bhardwaj, A.; Song, S.-J. A Facile Combustion Synthesis Route for Performance Enhancement of La0.6Sr0.4Co0.2Fe0.8O3 − δ (LSCF6428) as a Robust Cathode Material for IT-SOFC. J. Korean Ceram. Soc 2019, 56, 497–505. [Google Scholar] [CrossRef]
- Tai, X.Y.; Zhakeyev, A.; Wang, H.; Jiao, K.; Zhang, H.; Xuan, J. Accelerating Fuel Cell Development with Additive Manufacturing Technologies: State of the Art, Opportunities and Challenges. Fuel Cells 2019, 19, 636–650. [Google Scholar] [CrossRef]
- Minary-Jolandan, M. Formidable Challenges in Additive Manufacturing of Solid Oxide Electrolyzers (SOECs) and Solid Oxide Fuel Cells (SOFCs) for Electrolytic Hydrogen Economy toward Global Decarbonization. Ceramics 2022, 5, 761–779. [Google Scholar] [CrossRef]
- Zouridi, L.; Garagounis, I.; Vourros, A.; Marnellos, G.E.; Binas, V. Advances in Inkjet-Printed Solid Oxide Fuel Cells. Adv. Mater. Technol. 2022, 7, 2101491. [Google Scholar] [CrossRef]
- Kawale, S.S.; Jang, I.; Farandos, N.M.; Kelsall, G.H. Inkjet 3D-Printing of Functional Layers of Solid Oxide Electrochemical Reactors: A Review. React. Chem. Eng. 2022, 7, 1692–1712. [Google Scholar] [CrossRef]
- Jang, I.; Alexander, J.C.; Farandos, N.M.; Kelsall, G.H. Predicting Optimal Geometries of 3D-Printed Solid Oxide Electrochemical Reactors. Electrochim. Acta 2022, 427, 140902. [Google Scholar] [CrossRef]
- Rath, M.K.; Kossenko, A.; Danchuk, V.; Shatalov, M.; Rahumi, O.; Borodianskiy, K.; Zinigrad, M.; Sahoo, T.; Mishra, S. Development of Highly Efficient and Durable Large-Area Solid Oxide Fuel Cell by a Direct-Ink-Writing Three-Dimensional Printer. J. Power Sources 2022, 552, 232225. [Google Scholar] [CrossRef]
- Fashalameh, K.M.; Sadeghian, Z.; Ebrahimi, R. A High-Performance Planar Anode-Supported Solid Oxide Fuel Cell with Hierarchical Porous Structure through Slurry-Based Three-Dimensional Printing. J. Alloys Compd. 2022, 916, 165406. [Google Scholar] [CrossRef]
- Jang, I.; Kelsall, G.H. Fabrication of 3D NiO-YSZ Structures for Enhanced Performance of Solid Oxide Fuel Cells and Electrolysers. Electrochem. Commun. 2022, 137, 107260. [Google Scholar] [CrossRef]
- Farandos, N.M.; Jang, I.; Alexander, J.C.; Kelsall, G.H. 3-D Inkjet Printed Solid Oxide Electrochemical Reactors III. Cylindrical Pillared Electrode Microstructures. Electrochim. Acta 2022, 426, 140834. [Google Scholar] [CrossRef]
- Anelli, S.; Rosa, M.; Baiutti, F.; Torrell, M.; Esposito, V.; Tarancón, A. Hybrid-3D Printing of Symmetric Solid Oxide Cells by Inkjet Printing and Robocasting. Addit. Manuf. 2022, 51, 102636. [Google Scholar] [CrossRef]
- Tian, J.; Dou, Y.; Nian, Q.; Milcarek, R.J. Solid Oxide Fuel Cells with 3D Inkjet Printing Modified LSM−YSZ Interface. ECS Trans. 2022, 109, 51–61. [Google Scholar] [CrossRef]
- Lira, M.; Kostretsova, N.; Bernadet, L.; Morata, A.; Torrell, M.; Tarancón, A. Additive Manufacturing of Large Area SOC with Aadvanced Features. ECS Trans. 2021, 103, 149–157. [Google Scholar] [CrossRef]
- Qu, P.; Xiong, D.; Zhu, Z.; Gong, Z.; Li, Y.; Li, Y.; Fan, L.; Liu, Z.; Wang, P.; Liu, C.; et al. Inkjet Printing Additively Manufactured Multilayer SOFCs Using High Quality Ceramic Inks for Performance Enhancement. Addit. Manuf. 2021, 48, 102394. [Google Scholar] [CrossRef]
- Lee, J.; Byeon, J.; Lee, C. Fabrication of Thickness-Controllable Double Layer Electrolyte Using Roll-to-Roll Additive Manufacturing System. Int. J. Precis. Eng. Manuf. Green Technol. 2020, 7, 635–642. [Google Scholar] [CrossRef]
- Østergård, M.J.L.; Clausen, C.; Bagger, C.; Mogensen, M. Manganite-Zirconia Composite Cathodes for SOFC: Influence of Structure and Composition. Electrochim. Acta 1995, 40, 1971–1981. [Google Scholar] [CrossRef]
- Li, N. The Interaction of LSM−YSZ Composite and Improvement of the Solid Oxide Cell Durability by Mn-Modified YSZ. Ph.D. Thesis, University of Connecticut, Storrs, CT, USA, 2014. [Google Scholar]
- Natoli, A.; Arias-Serrano, B.I.; Rodríguez-Castellón, E.; Żurawska, A.; Frade, J.R.; Yaremchenko, A.A. Mixed Ionic-Electronic Conductivity, Redox Behavior and Thermochemical Expansion of Mn-Substituted 5YSZ as an Interlayer Material for Reversible Solid Oxide Cells. Materials 2021, 14, 641. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Feng, T.; Zhang, L.; Zhang, Y.; He, T. A-Site Deficient (La0.6Sr0.4)1 − xCo0.2Fe0.6Nb0.2O3 − δ Symmetrical Electrode Materials for Solid Oxide Fuel Cells. Electrochim. Acta 2018, 270, 174–182. [Google Scholar] [CrossRef]
- Bucher, E.; Sitte, W.; Klauser, F.; Bertel, E. Oxygen Exchange Kinetics of La0.6Sr0.4Co0.2Fe0.8O3 at 600 °C in Dry and Humid Atmospheres. Solid State Ion. 2011, 191, 61–67. [Google Scholar] [CrossRef]
- Kostogloudis, G.C.; Ftikos, C. Properties of A-Site-Deficient La0.6Sr0.4Co0.2Fe0.8O3 − δ-Based Perovskite Oxides. Solid State Ion. 1999, 126, 143–151. [Google Scholar] [CrossRef]
- Yang, Y.; Bao, H.; Ni, H.; Ou, X.; Wang, S.; Lin, B.; Feng, P.; Ling, Y. A Novel Facile Strategy to Suppress Sr Segregation for High-Entropy Stabilized La0.8Sr0.2MnO3 − δ Cathode. J. Power Sources 2021, 482, 228959. [Google Scholar] [CrossRef]
- Shi, Y.; Ni, N.; Ding, Q.; Zhao, X. Tailoring High-Temperature Stability and Electrical Conductivity of High Entropy Lanthanum Manganite for Solid Oxide Fuel Cell Cathodes. J. Mater. Chem. A 2022, 10, 2256–2270. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Olszewska, A.; Falkenstein, A.; Schwab, C.; Szymczak, M.; Zajusz, M.; Moździerz, M.; Mikuła, A.; Zielińska, K.; Berent, K.; et al. An Innovative Approach to Design SOFC Air Electrode Materials: High Entropy La1 − xSrx(Co,Cr,Fe,Mn,Ni)O3 − δ (x = 0, 0.1, 0.2, 0.3) Perovskites Synthesized by the Sol–Gel Method. J. Mater. Chem. A 2020, 8, 24455–24468. [Google Scholar] [CrossRef]
- Xu, H.; Dang, L.; Yan, J.; Wan, F.; Gong, W. A New (La0.2Nd0.2Gd0.2Sr0.2Ba0.2)Co0.2Fe0.8O3 − δ High-Entropy Oxide Cathode for Intermediate Temperature Solid Oxide Fuel Cell. Solid State Ion. 2023, 397, 116233. [Google Scholar] [CrossRef]
- Khan, M.S.; Xu, X.; Zhao, J.; Knibbe, R.; Zhu, Z. A Porous Yttria-Stabilized Zirconia Layer to Eliminate the Delamination of Air Electrode in Solid Oxide Electrolysis Cells. J. Power Sources 2017, 359, 104–110. [Google Scholar] [CrossRef]
- Wang, S. Promoting Effect of YSZ on the Electrochemical Performance of YSZ+LSM Composite Electrodes. Solid State Ion. 1998, 113–115, 291–303. [Google Scholar] [CrossRef]
- Rehman, S.U.; Song, R.-H.; Lee, J.-W.; Lim, T.-H.; Park, S.-J.; Lee, S.-B. Effect of GDC Addition Method on the Properties of LSM–YSZ Composite Cathode Support for Solid Oxide Fuel Cells. Ceram. Int. 2016, 42, 11772–11779. [Google Scholar] [CrossRef]
- Nielsen, J.; Hjelm, J. Impedance of SOFC Electrodes: A Review and a Comprehensive Case Study on the Impedance of LSM:YSZ Cathodes. Electrochim. Acta 2014, 115, 31–45. [Google Scholar] [CrossRef]
- Agbede, O.O.; Hellgardt, K.; Kelsall, G.H. Electrical Conductivities and Microstructures of LSM, LSM–YSZ and LSM–YSZ/LSM Cathodes Fabricated on YSZ Electrolyte Hollow Fibres by Dip-Coating. Mater. Today Chem. 2020, 16, 100252. [Google Scholar] [CrossRef]
- Chen, K.; Ai, N.; Jiang, S.P. Reasons for the High Stability of Nano-Structured (La,Sr)MnO3 Infiltrated Y2O3–ZrO2 Composite Oxygen Electrodes of Solid Oxide Electrolysis Cells. Electrochem. Commun. 2012, 19, 119–122. [Google Scholar] [CrossRef]
- Wang, J.X.; Sun, J.L.; He, C.R.; Wang, Q.; Wang, W.G. Mass Synthesis of High Performance (La0.75Sr0.25)0.95MnO3±δ Nano-Powder Prepared via a Low-Carbon Chemical Solution Method. J. Power Sources 2014, 253, 424–430. [Google Scholar] [CrossRef]
- Sun, K.; Piao, J.; Zhang, N.; Chen, X.; Xu, S.; Zhou, D. Fabrication and Performance of La0.8Sr0.2MnO3/YSZ Graded Composite Cathodes for SOFC. Rare Met. 2008, 27, 278–281. [Google Scholar] [CrossRef]
- Jørgensen, M. Effect of Sintering Temperature on Microstructure and Performance of LSM–YSZ Composite Cathodes. Solid State Ion. 2001, 139, 1–11. [Google Scholar] [CrossRef]
- Yeh, C.-Y.; Lin, T.-N.; Kuo, H.-Y.; Liao, M.-W.; Chen, Y.-M.; Kao, W.-X.; Lee, R.-Y.; Lee, S.-W. Microstructure Modification in the Electrodes to Enhance Performance of the Anode-Supported Solid Oxide Fuel Cell. In Ceramic Engineering and Science Proceedings; Salem, J., Koch, D., Mechnich, P., Kusnezoff, M., Bansal, N., LaSalvia, J., Balaya, P., Fu, Z., Ohji, T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 113–121. ISBN 978-1-119-54334-3. [Google Scholar]
- Taylor, T.H.; Muhoza, S.P.; Gross, M.D. Lowering the Impedance of Lanthanum Strontium Manganite-Based Electrodes with Lanthanum Oxychloride and Lanthanum Scavenging Chloride Salts. J. Electrochem. Soc. 2021, 168, 114508. [Google Scholar] [CrossRef]
- Lee, E.; Jeong, H.; Shin, T.H.; Myung, J.-H. Determination of the Rate-Determining Step of the Oxygen Reduction Reaction of La0.8Sr0.2MnO3(LSM)-8mol% Yttria-Stabilized Zirconia(YSZ): Composition and Microstructure. Ceram. Int. 2021, 47, 1792–1797. [Google Scholar] [CrossRef]
- Burmistrov, I.; Agarkov, D.; Bredikhin, S.; Nepochatov, Y.; Tiunova, O.; Zadorozhnaya, O. Multilayered Electrolyte-Supported SOFC Based on NEVZ-Ceramics Membrane. ECS Trans. 2013, 57, 917–923. [Google Scholar] [CrossRef]
- Khan, M.S.; Xu, X.; Knibbe, R.; Zhu, Z. Porous Scandia-Stabilized Zirconia Layer for Enhanced Performance of Reversible Solid Oxide Cells. ACS Appl. Mater. Interfaces 2018, 10, 25295–25302. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hanamura, K. Particle-Scaled Visualization of Active Sites in LSM/ScSZ Composite Cathode of SOFC through Oxygen Isotope Labeling. ECS Trans. 2017, 78, 855–859. [Google Scholar] [CrossRef]
- Yaroslavtsev, I.Y.; Bronin, D.I.; Vdovin, G.K.; Isupova, L.A. Oxide Cathodes for Electrochemical Devices Made with the Use of a Nanostructured Composition Material. Russ. J. Electrochem. 2012, 48, 981–985. [Google Scholar] [CrossRef]
- Sadykov, V.; Alikina, G.; Lukashevich, A.; Muzykantov, V.; Usoltsev, V.; Boronin, A.; Koscheev, S.; Krieger, T.; Ishchenko, A.; Smirnova, A.; et al. Design and Characterization of LSM/ScCeSZ Nanocomposite as Mixed Ionic–Electronic Conducting Material for Functionally Graded Cathodes of Solid Oxide Fuel Cells. Solid State Ion. 2011, 192, 540–546. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Bogdanovich, N.M.; Beresnev, S.M.; Pikalova, E.Y.; Bronin, D.I.; Zaikov, Y.P. Reversible Solid Oxide Fuel Cell for Power Accumulation and Generation. Russ. J. Electrochem. 2018, 54, 644–649. [Google Scholar] [CrossRef]
- Jeong, H.; Sharma, B.; Jo, S.; Kim, Y.H.; Myung, J. Electrochemical Characteristics of La0.8Sr0.2MnO3 (LSM)–Scandia-Stabilized Zirconia (ScSZ) Composite Cathode. J. Korean Ceram. Soc. 2022, 59, 473–479. [Google Scholar] [CrossRef]
- Sanna, C.; Squizzato, E.; Costamagna, P.; Holtappels, P.; Glisenti, A. Electrochemical Study of Symmetrical Intermediate Temperature—Solid Oxide Fuel Cells Based on La0.6Sr0.4MnO/Ce0.9Gd0.1O1.95 for Operation in Direct Methane/Air. Electrochim. Acta 2022, 409, 139939. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xu, Z.; Deng, H.; Dong, W.; Wang, B.; Feng, C.; Liu, X.; Wang, H. Semiconductor-Ionic Nanocomposite La0.1Sr0.9MnO3 − δ−Ce0.8Sm0.2O2 − δ Functional Layer for High Performance Low Temperature SOFC. Materials 2018, 11, 1549. [Google Scholar] [CrossRef]
- Baiutti, F.; Chiabrera, F.; Acosta, M.; Diercks, D.; Parfitt, D.; Santiso, J.; Wang, X.; Cavallaro, A.; Morata, A.; Wang, H.; et al. A High-Entropy Manganite in an Ordered Nanocomposite for Long-Term Application in Solid Oxide Cells. Nat. Commun. 2021, 12, 2660. [Google Scholar] [CrossRef]
- Pajot, M.; Duffort, V.; Capoen, E.; Mamede, A.-S.; Vannier, R.-N. Influence of the Strontium Content on the Performance of La1 − xSrxMnO3/Bi1.5Er0.5O3 Composite Electrodes for Low Temperature Solid Oxide Fuel Cells. J. Power Sources 2020, 450, 227649. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, K. A New Composite Cathode for Intermediate Temperature Solid Oxide Fuel Cells with Zirconia-Based Electrolytes. J. Power Sources 2017, 342, 419–426. [Google Scholar] [CrossRef]
- Timurkutluk, B.; Altan, T.; Toros, S.; Genc, O.; Celik, S.; Korkmaz, H.G. Engineering Solid Oxide Fuel Cell Electrode Microstructure by a Micro-Modeling Tool Based on Estimation of TPB Length. Int. J. Hydrogen Energy 2021, 46, 13298–13317. [Google Scholar] [CrossRef]
- Kim, S.J.; Dayaghi, A.M.; Kim, K.J.; Choi, G.M. Er0.4Bi1.6O3 − δ–La0.8Sr0.2MnO3 − δ Nano-Composite as a Low-Temperature Firing Cathode of Solid Oxide Fuel Cell. J. Power Sources 2017, 344, 218–222. [Google Scholar] [CrossRef]
- Lee, K.T.; Jung, D.W.; Yoon, H.S.; Camaratta, M.; Sexson, N.; Wachsman, E.D. High Performance LSM-ESB Cathode on ESB Electrolyte for Low to Intermediate Temperature Solid Oxide Fuel Cells. ECS Trans. 2011, 35, 1861–1869. [Google Scholar] [CrossRef]
- Yun, B.-H.; Kim, K.J.; Joh, D.W.; Chae, M.S.; Lee, J.J.; Kim, D.; Kang, S.; Choi, D.; Hong, S.-T.; Lee, K.T. Highly Active and Durable Double-Doped Bismuth Oxide-Based Oxygen Electrodes for Reversible Solid Oxide Cells at Reduced Temperatures. J. Mater. Chem. A 2019, 7, 20558–20566. [Google Scholar] [CrossRef]
- Dotelli, G.; Mari, C.; Ruffo, R.; Pelosato, R.; Sora, I. Electrical Behaviour of LSGM–LSM Composite Cathode Materials. Solid State Ion. 2006, 177, 1991–1996. [Google Scholar] [CrossRef]
- Pelosato, R.; Sora, I.N.; Dotelli, G.; Ruffo, R.; Mari, C.M. Characterization of (1 − x)La0.83Sr0.17Ga0.83Mg0.17O2.83−xLa0.8Sr0.2MnO3 (0 ≤ x ≤ 1) Composite Cathodes. J. Eur. Ceram. Soc. 2005, 25, 2587–2591. [Google Scholar] [CrossRef]
- Shahrokhi, S.; Babaei, A.; Zamani, C. Reversible Operation of La0.8Sr0.2MnO3 Oxygen Electrode Infiltrated with Ruddlesden-Popper and Perovskite Lanthanum Nickel Cobaltite. Int. J. Hydrogen Energy 2018, 43, 23091–23100. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, M.; Yang, L.; Liu, M. LSM-Infiltrated LSCF Cathodes for Solid Oxide Fuel Cells. J. Energy Chem. 2013, 22, 555–559. [Google Scholar] [CrossRef]
- Ai, N.; Chen, K.; Jiang, S.P. A La0.8Sr0.2MnO3/La0.6Sr0.4Co0.2Fe0.8O3 − δ Core-Shell Structured Cathode by a Rapid Sintering Process for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 7246–7251. [Google Scholar] [CrossRef]
- Seyed-Vakili, S.V.; Babaei, A.; Ataie, M.; Heshmati-Manesh, S.; Abdizadeh, H. Enhanced Performance of La0.8Sr0.2MnO3 Cathode for Solid Oxide Fuel Cells by Co-Infiltration of Metal and Ceramic Precursors. J. Alloys Compd. 2018, 737, 433–441. [Google Scholar] [CrossRef]
- Khellaf, N.; Kahoul, A.; Naamoune, F.; Cheriti, M. Impact of the Synthesis Method on Electrochemical Performance of La0.5Sr0.5MnO3 Perovskite Oxide as a Cathode Material in Alkaline Fuel Cells. Russ. J. Electrochem. 2022, 58, 10–20. [Google Scholar] [CrossRef]
- Leng, Y.; Chan, S.; Liu, Q. Development of LSCF–GDC Composite Cathodes for Low-Temperature Solid Oxide Fuel Cells with Thin Film GDC Electrolyte. Int. J. Hydrogen Energy 2008, 33, 3808–3817. [Google Scholar] [CrossRef]
- Liu, Q.L.; Khor, K.A.; Chan, S.H. Optimization of LSCF–GDC Composite Cathodes for Thin Film GDC Electrolyte Solid Oxide Fuel Cells. In Proceedings of the Third International Conference on Materials Processing for Properties and Performance (MP3), Singapore, 24–26 November 2004; Khor, K.A., Ramanujan, R.V., Ooi, C.P., Zhao, J., Eds.; Institute of Materials (East Asia): Singapore, 2004; pp. 657–666. [Google Scholar]
- Dusastre, V.; Kilner, J.A. Optimisation of Composite Cathodes for Intermediate Temperature SOFC Applications. Solid State Ion. 1999, 126, 163–174. [Google Scholar] [CrossRef]
- Perry Murray, E. Electrochemical Performance of (La,Sr)(Co,Fe)O3–(Ce,Gd)O3 Composite Cathodes. Solid State Ion. 2002, 148, 27–34. [Google Scholar] [CrossRef]
- Chen, J.; Liang, F.; Yan, D.; Pu, J.; Chi, B.; Jiang, S.P.; Jian, L. Performance of Large-Scale Anode-Supported Solid Oxide Fuel Cells with Impregnated La0.6Sr0.4Co0.2Fe0.8O3 − δ+Y2O3 Stabilized ZrO2 Composite Cathodes. J. Power Sources 2010, 195, 5201–5205. [Google Scholar] [CrossRef]
- Han, M.F.; Liu, Z.; Qian, J. Fabrication and Characterization of La0.6Sr0.4Co0.2Fe0.8O3-δ–Yttria Stabilized Zirconia Composites Cathode Prepared by Infiltration Method. ECS Trans. 2011, 35, 2295–2303. [Google Scholar] [CrossRef]
- Ko, H.J.; Myung, J.; Lee, J.-H.; Hyun, S.-H.; Chung, J.S. Synthesis and Evaluation of (La0.6Sr0.4)(Co0.2Fe0.8)O3 (LSCF)–Y0.08Zr0.92O1.96 (YSZ)–Gd0.1Ce0.9O2 − δ (GDC) Dual Composite SOFC Cathodes for High Performance and Durability. Int. J. Hydrogen Energy 2012, 37, 17209–17216. [Google Scholar] [CrossRef]
- Gong, J.; Shi, H.; Yu, Y.; Yue, Z.; Wang, Y.; Tan, W. Restrictions of Nitric Oxide Electrocatalytic Decomposition over Perovskite Cathode in Presence of Oxygen: Oxygen Surface Exchange and Diffusion. J. Colloid Interface Sci. 2022, 628, 95–105. [Google Scholar] [CrossRef]
- Xi, X.; Kondo, A.; Kozawa, T.; Naito, M. LSCF–GDC Composite Particles for Solid Oxide Fuel Cells Cathodes Prepared by Facile Mechanical Method. Adv. Powder Technol. 2016, 27, 646–651. [Google Scholar] [CrossRef]
- Gao, C.; Liu, Y.; Xi, K.; Jiao, S.; Tomov, R.I.; Kumar, R.V. Improve the Catalytic Property of La0.6Sr0.4Co0.2Fe0.8O3/Ce0.9Gd0.1O2 (LSCF/CGO) Cathodes with CuO Nanoparticles Infiltration. Electrochim. Acta 2017, 246, 148–155. [Google Scholar] [CrossRef]
- Zhao, E.; Liu, X.; Liu, L.; Huo, H.; Xiong, Y. Effect of La0.8Sr0.2Co0.2Fe0.8O3 − δ Morphology on the Performance of Composite Cathodes. Progr. Nat. Sci. Mater. Inter. 2014, 24, 24–30. [Google Scholar] [CrossRef]
- Zhao, L.; Amarasinghe, S.; Jiang, S.P. Enhanced Chromium Tolerance of La0.6Sr0.4Co0.2Fe0.8O3 − δ Electrode of Solid Oxide Fuel Cells by Gd0.1Ce0.9O1.95 Impregnation. Electrochem. Commun. 2013, 37, 84–87. [Google Scholar] [CrossRef]
- Li, N.; Verma, A.; Singh, P.; Kim, J.-H. Characterization of La0.58Sr0.4Co0.2Fe0.8O3 − δ–Ce0.8Gd0.2O2 Composite Cathode for Intermediate Temperature Solid Oxide Fuel Cells. Ceram. Int. 2013, 39, 529–538. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Ni, D.; Sun, K. Preparation of Honeycomb Porous La0.6Sr0.4Co0.2Fe0.8O3 − δ–Gd0.2Ce0.8O2 − δ Composite Cathodes by Breath Figures Method for Solid Oxide Fuel Cells. Appl. Surf. Sci. 2011, 258, 50–57. [Google Scholar] [CrossRef]
- Zhi, M.; Lee, S.; Miller, N.; Menzler, N.H.; Wu, N. An Intermediate-Temperature Solid Oxide Fuel Cell with Electrospun Nanofiber Cathode. Energy Environ. Sci. 2012, 5, 7066. [Google Scholar] [CrossRef]
- Shen, F.; Lu, K. La0.6Sr0.4Co0.2Fe0.8O3 Cathodes Incorporated with Sm0.2Ce0.8O2 by Three Different Methods for Solid Oxide Fuel Cells. J. Power Sources 2015, 296, 318–326. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Xia, C. Oxygen Incorporation at the Three-Phase Boundary of LSCF–SDC Composite. J. Power Sources 2014, 269, 180–188. [Google Scholar] [CrossRef]
- Lee, S.; Song, H.S.; Hyun, S.H.; Kim, J.; Moon, J. LSCF–SDC Core–Shell High-Performance Durable Composite Cathode. J. Power Sources 2010, 195, 118–123. [Google Scholar] [CrossRef]
- Nie, L.; Liu, M.; Zhang, Y.; Liu, M. La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes Infiltrated with Samarium-Doped Cerium Oxide for Solid Oxide Fuel Cells. J. Power Sources 2010, 195, 4704–4708. [Google Scholar] [CrossRef]
- Wang, S. Performance of a La0.6Sr0.4Co0.2Fe0.8O3–Ce0.8Gd0.2O1.9–Ag Cathode for Ceria Electrolyte SOFCs. Solid State Ion. 2002, 146, 203–210. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Z.; Tu, B.; Cui, D.; Ou, D.; Cheng, M. Enhanced Performance and Stability of Interlayer-Free La0.6Sr0.4Co0.2Fe0.8O3 − δ−Ce0.8Zr0.2O2 − δ Cathode for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2015, 40, 4861–4867. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Jin, C.; Gao, H.; Zhang, Y. Electrochemical Evaluation of La0.6Sr0.4Co0.2Fe0.8O3 − δ–La0.9Sr0.1Ga0.8Mg0.2O3 − δ Composite Cathodes for La0.9Sr0.1Ga0.8Mg0.2O3 − δ Electrolyte SOFCs. J. Alloys Compd. 2009, 473, 43–47. [Google Scholar] [CrossRef]
- Lin, Y.; Barnett, S. La0.9Sr0.1Ga0.8Mg0.2O3 − δ−La0.6Sr0.4Co0.2Fe0.8O3 − δ Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. Solid State Ion. 2008, 179, 420–427. [Google Scholar] [CrossRef]
- Giuliano, A.; Carpanese, M.P.; Panizza, M.; Cerisola, G.; Clematis, D.; Barbucci, A. Characterisation of La0.6Sr0.4Co0.2Fe0.8O3 − δ–Ba0.5Sr0.5Co0.8Fe0.2O3 − δ Composite as Cathode for Solid Oxide Fuel Cells. Electrochim. Acta 2017, 240, 258–266. [Google Scholar] [CrossRef]
- Świerczek, K. Electrolyte-Supported IT-SOFC with LSCF–SCFN Composite Cathode. Solid State Ion. 2011, 192, 486–490. [Google Scholar] [CrossRef]
- Burye, T.E.; Nicholas, J.D. Improving La0.6Sr0.4Co0.2Fe0.8O3 − δ Infiltrated Solid Oxide Fuel Cell Cathode Performance through Precursor Solution Desiccation. J. Power Sources 2015, 276, 54–61. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Geng, C.; Cao, H.; Xu, C.; Cheng, J.; Hong, T. A Novel Core-Shell LSCF Perovskite Structured Electrocatalyst with Local Hetero-Interface for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 11824–11833. [Google Scholar] [CrossRef]
- Fasquelle, D.; Chi, Z.; Belakry, S. Impregnation of Gadolinium-Doped Ceria Backbone Electrodes Modified by Addition of Pore-Formers for SOFC Application. J. Solid State Electrochem. 2023, 27, 695–703. [Google Scholar] [CrossRef]
- Wei, M.; Chen, H.; Zhao, X.; Liu, Y.; Chen, X.; Li, W.; Kang, K.; Wang, C. High Performance La2NiO4+δ Impregnated La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathodes for Solid Oxide Fuel Cells. Solid State Ion. 2022, 387, 116065. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Z.; Zhao, X.; Zhong, S.; Chen, X.; Wang, C. Performance of PrBaCo2O5+δ Impregnated La0.6Sr0.4Co0.2Fe0.8O3 − δ Composites as Potential Cathode Materials for Solid Oxide Fuel Cells. J. Alloys Compd. 2021, 886, 161155. [Google Scholar] [CrossRef]
- Zheng, Z.; Jing, J.; Yu, H.; Yang, Z.; Jin, C.; Chen, F.; Peng, S. Boosting and Robust Multifunction Cathode Layer for Solid Oxide Fuel Cells. ACS Sustain. Chem. Eng. 2022, 10, 6817–6825. [Google Scholar] [CrossRef]
- Wang, J.; Yang, T.; Wen, Y.; Zhang, Y.; Sun, C.; Huang, K. Performance and Stability of SrCo0.9Nb0.1O3 − δ–(La0.60Sr0.40)0.95(Co0.20Fe0.80)O3 − δ Bilayer Cathode for Intermediate-Temperature Solid Oxide Fuel Cells. J. Power Sources 2019, 414, 24–30. [Google Scholar] [CrossRef]
- Chen, J.; Liang, F.; Chi, B.; Pu, J.; Jiang, S.P.; Jian, L. Palladium and Ceria Infiltrated La0.8Sr0.2Co0.5Fe0.5O3 − δ Cathodes of Solid Oxide Fuel Cells. J. Power Sources 2009, 194, 275–280. [Google Scholar] [CrossRef]
- Ji, Y.; Kilner, J.; Carolan, M. Electrical Properties and Oxygen Diffusion in Yttria-Stabilised Zirconia (YSZ)–La0.8Sr0.2MnO3±δ (LSM) Composites. Solid State Ion. 2005, 176, 937–943. [Google Scholar] [CrossRef]
- Agbede, O.O.; Kelsall, G.H.; Hellgardt, K. A Solid Oxide Fuel Cell with Molten Tin Anode for Electricity Generation and Methane Reforming. J. Power Sources 2020, 474, 228577. [Google Scholar] [CrossRef]
- Harboe, S.; Sohn, Y.J.; Guillon, O.; Menzler, N.H. Investigation of LSM–8YSZ Cathode within an All Ceramic SOFC. Part I: Chemical Interactions. J. Eur. Ceram. Soc. 2020, 40, 3608–3617. [Google Scholar] [CrossRef]
- Matte, E.; Holzlechner, G.; Epple, L.; Stolten, D.; Lupetin, P. Impact of Silicate Substrate and Cosintering on Cathode Performance in an Inert Substrate-Supported Solid Oxide Fuel Cell. J. Power Sources 2019, 413, 334–343. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Y.; Zhou, X.; Kong, X.; Zhang, J.; Zuo, W.; Ye, X. High Performance Yttria-Stabilized Zirconia Based Intermediate Temperature Solid Oxide Fuel Cells with Double Nano Layer Composite Cathode. Int. J. Hydrogen Energy 2017, 42, 1093–1102. [Google Scholar] [CrossRef]
- Celikbilek, O.; Dessemond, L.; Djurado, E. State-of-the-Art La0.6Sr0.4Co0.2Fe0.8O3 − δ Cathode for SOFC: Microstructural and Electrochemical Properties. ECS Trans. 2017, 78, 747–758. [Google Scholar] [CrossRef]
- Sohal, M.S.; O’Brien, J.E.; Stoots, C.M.; Sharma, V.I.; Yildiz, B.; Virkar, A. Degradation Issues in Solid Oxide Cells during High Temperature Electrolysis. In Proceedings of the ASME 2010 8th International Fuel Cell Science, Engineering and Technology Conference, Brooklyn, New York, NY, USA, 1 January 2010; ASMEDC: Houston, TX, USA, 2010; Volume 1, pp. 377–387. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, Q.; Yan, Z.; Jiao, Z.; Bi, L.; Chan, S.H.; Zhong, Z. On the Delamination of Air Electrodes of Solid Oxide Electrolysis Cells: A Mini-Review. Electrochem. Commun. 2022, 137, 107267. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.W.; Park, Y.M.; Kim, K.J.; Choi, G.M. Effect of Gd-Doped Ceria Interlayer on the Stability of Solid Oxide Electrolysis Cell. Solid State Ion. 2016, 295, 25–31. [Google Scholar] [CrossRef]
- Jang, I.; Kim, S.; Kim, C.; Lee, H.; Yoon, H.; Song, T.; Paik, U. Interface Engineering of Yttrium Stabilized Zirconia/Gadolinium Doped Ceria Bi-Layer Electrolyte Solid Oxide Fuel Cell for Boosting Electrochemical Performance. J. Power Sources 2019, 435, 226776. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, K.J.; Choi, G.M. Effect of Ce0.43Zr0.43Gd0.1Y0.04O2 − δ Contact Layer on Stability of Interface between GDC Interlayer and YSZ Electrolyte in Solid Oxide Electrolysis Cell. J. Power Sources 2015, 284, 617–622. [Google Scholar] [CrossRef]
- Ni, N.; Xiao, W.; Zheng, C.; Jiang, J.; Yu, Y.; Hao, W.; Tang, M.; Shen, H.; Peng, D. Flash Cosintering of a Lanthanum Strontium Cobalt Ferrite Nanofibre/Gd-Doped Ceria Bilayer Structure. J. Eur. Ceram. Soc. 2022, 42, 2870–2878. [Google Scholar] [CrossRef]
- He, A.; Onishi, J.; Gong, J.; Shikazono, N. Three-Dimensional Optimization of La0.6Sr0.4Co0.2Fe0.8O3 Cathode Microstructure with Particle Radius Constraint. Electrochim. Acta 2021, 398, 139287. [Google Scholar] [CrossRef]
- He, A.; Onishi, J.; Gong, J.; Shikazono, N. Microstructure Optimization of Porous Mixed Ionic and Electronic Conducting Cathode for Solid Oxide Fuel Cells. J. Power Sources 2020, 478, 228771. [Google Scholar] [CrossRef]
- He, A.; Onishi, J.; Shikazono, N. Numerical Optimization of the Solid Oxide Fuel Cell Electrode-Electrolyte Interface Structure with Adjoint Method. In Proceedings of the ECS Transactions; Eguchi, K., Singhal, S.C., Eds.; Electrochemical Society Inc.: Philadelphia, PA, USA, 2019; Volume 91, pp. 2045–2054. [Google Scholar] [CrossRef]
- Sharma, S.; Stanley, R.; Kumari, N. Electrochemical Modeling and Simulation of CO2 Reduction in Solid Oxide Electrolysis Cells. J. Hazard. Toxic Radioact. Waste 2022, 26, 04022005. [Google Scholar] [CrossRef]
- Yang, T.; Liu, J.; Lei, Y.; Li, W.; Cheng, T.-L.; Finklea, H.; Wen, Y.-H.; Liu, X.; Abernathy, H.W.; Lee, S.; et al. Investigation of LSM−YSZ Composite Cathode Performance Degradation with a Multistep Charge Transfer Model. J. Electrochem. Soc. 2019, 166, F448–F457. [Google Scholar] [CrossRef]
- Ananyev, M.V.; Farlenkov, A.S.; Eremin, V.A.; Kurumchin, E.K. Degradation Kinetics of LSM–YSZ Cathode Materials for SOFC. Int. J. Hydrogen Energy 2018, 43, 951–959. [Google Scholar] [CrossRef]
- Cook, K.; Wrubel, J.; Ma, Z.; Huang, K.; Jin, X. Modeling Electrokinetics of Oxygen Electrodes in Solid Oxide Electrolyzer Cells. J. Electrochem. Soc. 2021, 168, 114510. [Google Scholar] [CrossRef]
- Yan, Z.; He, A.; Hara, S.; Shikazono, N. Modeling of Solid Oxide Fuel Cell (SOFC) Electrodes from Fabrication to Operation: Correlations between Microstructures and Electrochemical Performances. Energy Convers. Manag. 2019, 190, 1–13. [Google Scholar] [CrossRef]
- He, A.; Shimura, T.; Gong, J.; Shikazono, N. Numerical Simulation of La0.6Sr0.4Co0.2Fe0.8O3−Gd0.1Ce0.9O1.95 Composite Cathodes with Micro Pillars. Int. J. Hydrogen Energy 2019, 44, 6871–6885. [Google Scholar] [CrossRef]
- Subramanian, Y.; Veena, R.; Muhammed Ali, S.A.; Kumar, A.; Gubediran, R.K.; Dhanasekaran, A.; Gurusamy, D.; Muniandi, K. Artificial Intelligence Technique Based Performance Estimation of Solid Oxide Fuel Cells. Mater. Today Proc. 2023, 80, 2573–2576. [Google Scholar] [CrossRef]
- Donazzi, A.; De Pascali, S.; Garavaglia, F.; Bracconi, M. A Quasi 2D Model for the Interpretation of Impedance and Polarization of a Planar Solid Oxide Fuel Cell with Interconnects. Electrochim. Acta 2021, 365, 137346. [Google Scholar] [CrossRef]
- Iwai, H.; Maejima, K.; Kishimoto, M.; Saito, M.; Yoshida, H.; Kishimoto, H.; Yamaji, K.; Yokokawa, H. Numerical Model of Sulfur Poisoning on LSCF Cathode and Its Application to a 2-D Single Cell Simulation. In Proceedings of the ECS Transactions; Eguchi, K., Singhal, S.C., Eds.; Electrochemical Society Inc.: Philadelphia, PA, USA, 2019; Volume 91, pp. 2115–2125. [Google Scholar]
- Effori, E.; Laurencin, J.; Tezyk, V.; Montella, C.; Dessemond, L.; Siebert, E. A Physically-Based Modelling to Predict the Cyclic Voltammetry Response of LSCF-Type Electrodes: Impact of the Ohmic Losses and Microstructure. Solid State Ion. 2021, 371, 115765. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filonova, E.; Pikalova, E. Overview of Approaches to Increase the Electrochemical Activity of Conventional Perovskite Air Electrodes. Materials 2023, 16, 4967. https://doi.org/10.3390/ma16144967
Filonova E, Pikalova E. Overview of Approaches to Increase the Electrochemical Activity of Conventional Perovskite Air Electrodes. Materials. 2023; 16(14):4967. https://doi.org/10.3390/ma16144967
Chicago/Turabian StyleFilonova, Elena, and Elena Pikalova. 2023. "Overview of Approaches to Increase the Electrochemical Activity of Conventional Perovskite Air Electrodes" Materials 16, no. 14: 4967. https://doi.org/10.3390/ma16144967
APA StyleFilonova, E., & Pikalova, E. (2023). Overview of Approaches to Increase the Electrochemical Activity of Conventional Perovskite Air Electrodes. Materials, 16(14), 4967. https://doi.org/10.3390/ma16144967