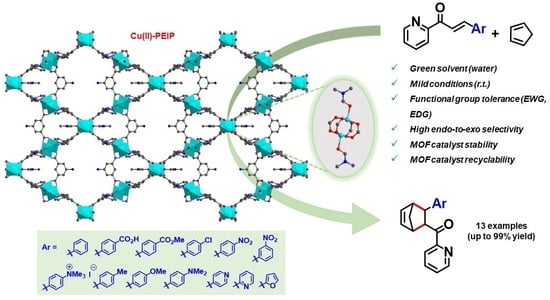
Catalysis of a Diels–Alder Reaction between Azachalcones and Cyclopentadiene by a Recyclable Copper(II)-PEIP Metal-Organic Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Cu(II)-PEIP MOF Preparation
2.3. Synthesis of Azachalcone Dienophile Building Units
2.4. Screening of Conditions for [4 + 2] Cycloaddition (Diels–Alder Reaction)
2.5. Control Experiments for Assessing the Effect of Individual MOF Components in Catalysis and Control Reactions with a Homogeneous Copper(II) Catalyst, Cu(OTf)2
2.6. Procedure for Cu(II)-PEIP MOF-Catalyzed [4 + 2] (Diels–Alder) Cycloaddition
2.7. Assessment of MOF’s Structural Integrity under D-A Conditions, and after each Catalytic Cycle
3. Results and Discussion
3.1. Suitability of Cu(II)-PEIP MOF for Catalysis
3.2. Selection of Reaction Solvent for D-A Cycloaddition
3.3. Ability of Individual Components of Cu(II)-PEIP MOF to Catalyze a Model D-A Reaction
3.4. Investigation of Azachalcone Dienophile Scope in Cu(II)-PEIP-Catalyzed D-A
3.5. Recyclability of Cu(II)-PEIP Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Zulkifli, Z.I.; Lim, K.L.; Teh, L.P. Metal-Organic Frameworks (MOFs) and Their Applications in CO2 Adsorption and Conversion. ChemistrySelect 2022, 7, e202200572. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A Historical Overview of the Activation and Porosity of Metal–Organic Frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhao, D. Metal–Organic Frameworks with Lewis Acidity: Synthesis, Characterization, and Catalytic Applications. CrystEngComm 2017, 19, 4066–4081. [Google Scholar] [CrossRef]
- Morris, R.E.; Wheatley, P.S. Gas Storage in Nanoporous Materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent Advances in Gas Storage and Separation Using Metal–Organic Frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Kourtellaris, A.; Moushi, E.E.; Spanopoulos, I.; Trikalitis, P.N.; Pissas, M.; Papaefstathiou, G.S.; Sanakis, Y.; Tasiopoulos, A.J. A Microporous Co(II)-Based 3-D Metal Organic Framework Built from Magnetic Infinite Rod-Shaped Secondary Building Units. Eur. J. Inorg. Chem. 2019, 2019, 4056–4062. [Google Scholar] [CrossRef]
- Panagiotou, N.; Moscoso, F.G.; Lopes-Costa, T.; Pedrosa, J.M.; Tasiopoulos, A.J. 2-Dimensional Rare Earth Metal–Organic Frameworks Based on a Hexanuclear Secondary Building Unit as Efficient Detectors for Vapours of Nitroaromatics and Volatile Organic Compounds. Inorg. Chem. Front. 2022, 9, 4850–4863. [Google Scholar] [CrossRef]
- Kourtellaris, A.; Lafargue-Dit-Hauret, W.; Massuyeau, F.; Latouche, C.; Tasiopoulos, A.J.; Serier-Brault, H. Tuning of Thermometric Performances of Mixed Eu–Tb Metal–Organic Frameworks through Single-Crystal Coordinating Solvent Exchange Reactions. Adv. Opt. Mater. 2022, 10, 2200484. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and Photocatalysis by Metal Organic Frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [Green Version]
- Cirujano, F.G.; Martin, N.; Wee, L.H. Design of Hierarchical Architectures in Metal–Oganic Frameworks for Catalysis and Adsorption. Chem. Mater. 2020, 32, 10268–10295. [Google Scholar] [CrossRef]
- Gao, F.; Yan, R.; Shu, Y.; Cao, Q.; Zhang, L. Strategies for the Application of Metal–Organic Frameworks in Catalytic Reactions. RSC Adv. 2022, 12, 10114–10125. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xu, H.; Jiang, N.; Wang, M.; Huang, J.; Guan, L. Tensile-Strained RuO2 Loaded on Antimony-Tin Oxide by Fast Quenching for Proton-Exchange Membrane Water Electrolyzer. Adv. Sci. 2022, 9, 2201654. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously Mastering Operando Strain and Reconstruction Effects via Phase-Segregation Strategy for Enhanced Oxygen-Evolving Electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Zhao, S. A Novel 3D MOF with Rich Lewis Basic Sites as a Base Catalysis toward Knoevenagel Condensation Reaction. J. Mol. Struct. 2018, 1167, 11–15. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Heidenreich, N.; Lenzen, D.; Stock, N. Knoevenagel Condensation Reaction Catalysed by Al-MOFs with CAU-1 and CAU-10-Type Structures. CrystEngComm 2017, 19, 4187–4193. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Le, K.K.A.; Phan, T.D. MOF-5 as an Efficient Heterogeneous Catalyst for Friedel–Crafts Alkylation Reactions. Appl. Catal. Gen. 2010, 382, 246–253. [Google Scholar] [CrossRef]
- Ghosh, S.; Nagarjun, N.; Alam, M.; Dhakshinamoorthy, A.; Biswas, S. Friedel-Crafts Alkylation Reaction Efficiently Catalyzed by a Di-Amide Functionalized Zr(IV) Metal-Organic Framework. Mol. Catal. 2022, 517, 112007. [Google Scholar] [CrossRef]
- Diyali, N.; Rasaily, S.; Biswas, B. Metal–Organic Framework: An Emergent Catalyst in C–N Cross-Coupling Reactions. Coord. Chem. Rev. 2022, 469, 214667. [Google Scholar] [CrossRef]
- Hoffmann, P.; Lherbet, C.; Fabing, I.; Barthélémy, M.-C.; Borjon-Piron, Y.; Laurent, C.; Vigroux, A. A Mesoporous Metal–Organic Framework used to Sustainably Release Copper(ii) into Reducing Aqueous Media to Promote the CuAAC Click Reaction. RSC Adv. 2022, 12, 26825–26833. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.-B.; Yang, J.; Che, G.-B.; Pei, W.-Y.; Ma, J.-F. Highly Stable Copper(I)-Based Metal−Organic Framework Assembled with Resorcin[4]arene and Polyoxometalate for Efficient Heterogeneous Catalysis of Azide−Alkyne “Click” Reaction. ACS Appl. Mater. Interfaces 2018, 10, 2628–2636. [Google Scholar] [CrossRef]
- Qi, C.; Ramella, D.; Wensley, A.M.; Luan, Y. A Metal-Organic Framework Brønsted Acid Catalyst: Synthesis, Characterization and Application to the Generation of Quinone Methides for [4 + 2] Cycloadditions. Adv. Synth. Catal. 2016, 358, 2604–2611. [Google Scholar] [CrossRef]
- Tanaka, K.; Nagase, S.; Anami, T.; Wierzbicki, M.; Urbanczyk-Lipkowska, Z. Enantioselective Diels–Alder Reaction in the Confined Space of Homochiral Metal–Organic Frameworks. RSC Adv. 2016, 6, 111436–111439. [Google Scholar] [CrossRef] [Green Version]
- Abbasnia, M.; Sheykhan, M.; Bahmani, M.; Taghizadeh, P. MOFs Come up to Scratch: An Environmentally Benign Route to Oxidative [4 + 2] Cycloaddition on Maleimides Solely via MOFs in Water. Green Chem. 2020, 22, 3216–3228. [Google Scholar] [CrossRef]
- Rechac, V.L.; Cirujano, F.G.; Corma, A.; Llabrés i Xamena, F.X. Diastereoselective Synthesis of Pyranoquinolines on Zirconium-Containing UiO-66 Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2016, 2016, 4512–4516. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.-Y.; Chen, Y.-P.; Park, J.; Wei, Z.; Sun, Y.; Bosch, M.; Yuan, S.; Zhou, H.-C. A Highly Stable Porphyrinic Zirconium Metal–Organic Framework with Shp-a Topology. J. Am. Chem. Soc. 2014, 136, 17714–17717. [Google Scholar] [CrossRef]
- Johnson, J.A.; Petersen, B.M.; Kormos, A.; Echeverría, E.; Chen, Y.-S.; Zhang, J. A New Approach to Non-Coordinating Anions: Lewis Acid Enhancement of Porphyrin Metal Centers in a Zwitterionic Metal–Organic Framework. J. Am. Chem. Soc. 2016, 138, 10293–10298. [Google Scholar] [CrossRef]
- Yang, L.; Cai, P.; Zhang, L.; Xu, X.; Yakovenko, A.A.; Wang, Q.; Pang, J.; Yuan, S.; Zou, X.; Huang, N.; et al. Ligand-Directed Conformational Control over Porphyrinic Zirconium Metal-Organic Frameworks for Size-Selective Catalysis. J. Am. Chem. Soc. 2021, 143, 12129–12137. [Google Scholar] [CrossRef]
- Gregoritza, M.; Brandl, F.P. The Diels–Alder Reaction: A Powerful Tool for the Design of Drug Delivery Systems and Biomaterials. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. [Google Scholar] [CrossRef]
- Funel, J.-A.; Abele, S. Industrial Applications of the Diels–Alder Reaction. Angew. Chem. Int. Ed. 2013, 52, 3822–3863. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Munakata, R.; Tadano, K. Recent Advances in Natural Product Synthesis by Using Intramolecular Diels−Alder Reactions. Chem. Rev. 2005, 105, 4779–4807. [Google Scholar] [CrossRef]
- Hsung, R.P.; Ma, Z.-X.; Fang, L.; Feltenberger, J.B. Diels–Alder Cascades in Natural Product Total Synthesis. In Efficiency in Natural Product Total Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 159–189. ISBN 978-1-118-94022-8. [Google Scholar]
- Vermeeren, P.; Hamlin, T.A.; Fernández, I.; Bickelhaupt, F.M. How Lewis Acids Catalyze Diels–Alder Reactions. Angew. Chem. Int. Ed. 2020, 59, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Kourtellaris, A.; Moushi, E.E.; Spanopoulos, I.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.A.; Papaefstathiou, G.S.; Trikalitis, P.N.; Tasiopoulos, A.J. A Microporous Cu2+ MOF Based on a Pyridyl Isophthalic Acid Schiff Base Ligand with High CO2 Uptake. Inorg. Chem. Front. 2016, 3, 1527–1535. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Mondal, S.; Patra, A.; Gonnade, R.G.; Biju, A.T. N-Heterocyclic Carbene-Catalyzed Diastereoselective Synthesis of β-Lactone-Fused Cyclopentanes Using Homoenolate Annulation Reaction. Chem. Commun. 2015, 51, 9559–9562. [Google Scholar] [CrossRef]
- Basler, S.; Studer, S.; Zou, Y.; Mori, T.; Ota, Y.; Camus, A.; Bunzel, H.A.; Helgeson, R.C.; Houk, K.N.; Jiménez-Osés, G.; et al. Efficient Lewis-Acid Catalysis of an Abiological Reaction in a De Novo Protein Scaffold. Nat. Chem. 2021, 13, 231–235. [Google Scholar] [CrossRef]
- Mukkala, V.-M.; Helenius, M.; Hemmilä, I.; Kankare, J.; Takalo, H. Development of Luminescent Europium(III) and Terbium(III) Chelates of 2,2′:6′,2″-Terpyridine Derivatives for Protein Labeling. Helv. Chim. Acta 1993, 76, 1361–1378. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Y.-W.; Wang, G.-W. Efficient and Clean Aldol Condensation Catalyzed by Sodium Carbonate in Water. Chem. Lett. 2003, 32, 966–967. [Google Scholar] [CrossRef]
- Fukui, K. Theory of Orientation and Stereoselection; Springer: Berlin/Heidelberg, Germany, 1975. [Google Scholar] [CrossRef]
- Jiang, J.; Meng, Y.; Zhang, L.; Liu, M. Self-Assembled Single-Walled Metal-Helical Nanotube (M-HN): Creation of Efficient Supramolecular Catalysts for Asymmetric Reaction. J. Am. Chem. Soc. 2016, 138, 15629–15635. [Google Scholar] [CrossRef] [PubMed]





| Entry | Solvent/Additive | Solubility of Cu(II)-PEIP | Conversion (%) |
|---|---|---|---|
| 1 | CH3CN | Insoluble | 8 |
| 2 | CH3CN/H2O (1:10 v/v) | Insoluble | 63 |
| 3 | H2O | Insoluble | 99 |
| 4 | DCM | Insoluble | 29 |
| 5 | MeOH | Insoluble | 28 |
| 6 | Sat. aqueous NaCl | Insoluble | 10 |
| 7 | NMP | Insoluble | trace |
| 8 | BME | Insoluble | 7 |
| 9 | EtOH | Insoluble | 10 |
| 10 | DMF | Insoluble | trace |
| 11 | H2O/25% mol SDS | Insoluble | 99 |
| 12 | H2O/10% mol SDS | Insoluble | 99 |
| 13 | H2O/5% mol SDS | Insoluble | 99 |
| Entry | MOF Component | Conversion (%) | Endo:Exo |
|---|---|---|---|
| 1 | Cu(NO3)2·2.5H2O | 35 | 1:1 |
| 2 | PEIPH2 | 25 | 1:1 |
| 3 | 5-NH2-mBDCH2 | 29 | 1:1 |
| 4 | None | trace | N/D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjikyprianou, E.; Petrides, S.; Kourtellaris, A.; Tasiopoulos, A.J.; Georgiades, S.N. Catalysis of a Diels–Alder Reaction between Azachalcones and Cyclopentadiene by a Recyclable Copper(II)-PEIP Metal-Organic Framework. Materials 2023, 16, 5298. https://doi.org/10.3390/ma16155298
Hadjikyprianou E, Petrides S, Kourtellaris A, Tasiopoulos AJ, Georgiades SN. Catalysis of a Diels–Alder Reaction between Azachalcones and Cyclopentadiene by a Recyclable Copper(II)-PEIP Metal-Organic Framework. Materials. 2023; 16(15):5298. https://doi.org/10.3390/ma16155298
Chicago/Turabian StyleHadjikyprianou, Eleni, Sotiris Petrides, Andreas Kourtellaris, Anastasios J. Tasiopoulos, and Savvas N. Georgiades. 2023. "Catalysis of a Diels–Alder Reaction between Azachalcones and Cyclopentadiene by a Recyclable Copper(II)-PEIP Metal-Organic Framework" Materials 16, no. 15: 5298. https://doi.org/10.3390/ma16155298
APA StyleHadjikyprianou, E., Petrides, S., Kourtellaris, A., Tasiopoulos, A. J., & Georgiades, S. N. (2023). Catalysis of a Diels–Alder Reaction between Azachalcones and Cyclopentadiene by a Recyclable Copper(II)-PEIP Metal-Organic Framework. Materials, 16(15), 5298. https://doi.org/10.3390/ma16155298