A Novel PLLA/MgF2 Coating on Mg Alloy by Ultrasonic Atomization Spraying for Controlling Degradation and Improving Biocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Mg Substrate
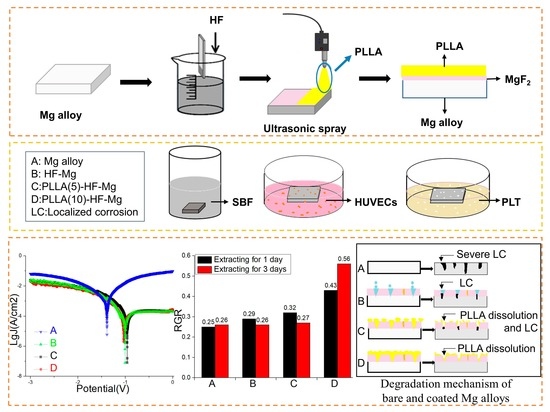
2.2. Fabrication of MgF2 and Polymer Coatings
2.3. Surface Characterization
2.4. In Vitro Biodegradation
2.5. Cell Culture and Tests
2.6. Platelet Adhesion Test
3. Results
3.1. Surface Morphology of Coated and Bare Mg Alloy Samples
3.2. In Vitro Degradation
3.2.1. Electrochemical Test
3.2.2. Immersion Test
3.3. Cell Culture and Test
3.3.1. Cell Adhesion and Proliferation
3.3.2. Cell Vitality
3.4. Platelet Adhesion Test
4. Discussions
4.1. The Novel Composite Coating Controls the Degradation Rate of Mg Alloy
4.2. The Novel Composite Coating Improves Biocompatibility of Mg Alloy
5. Conclusions
- The MgF2 layer was smooth and compact, but had some holes. The PLLA coating could partly cover these holes, providing a good physical barrier and reducing the corrosion of the Mg alloy substrate. The PLLA coating with five layers was uneven and could not cover the surface. However, the PLLA coating with 10 layers could almost cover the surface without excessive droplet overlap, mechanically locked with the substrate.
- Compared with the Icorr of the bare Mg alloy (9.58 × 10−4 A/mm2), the Icorr of PLLA (10)-HF-Mg (7.0 × 10−5 A/mm2) was significantly decreased, indicating a great improvement in corrosion resistance. While all the samples were immersed in SBF for 14 days, PLLA (10)-HF-Mg also had the best corrosion resistance. There were nearly no cracks on PLLA (10)-HF-Mg and some corrosion cracks on PLLA (5)-HF-Mg. However, the cracks on the bare Mg alloy were numerous and deep.
- HUVECs barely grew on the bare Mg alloy surface, but grew very well on the PLLA (10)-HF-Mg surface, indicating the good biocompatibility and endothelialization of PLLA (10)-HF-Mg. Meanwhile, the RGR of HUVECs in the PLLA (10)-HF-Mg extract significantly increased by 73% (1 day) and 111% (3 days).
- With the new composite PLLA/MgF2 coating, the number of adherent platelets was decreased. The adherent platelets showed a nearly round shape, which indicates less activation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The writing committee of the report on cardiovascular health diseases in China. Chin. Circ. J. 2020, 35, 833–854.
- Fontes, I.; Barrow, D. From sirolimus to the Cypher stent: Stages of the victory against restenosis. Ann. Cardiol. Angeiol. 2004, 53, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dorr, O.; Liebetrau, C.; Wiebe, J.; Hecker, F.; Rixe, J.; Mollmann, H.; Hamm, C.; Nef, H. Bioresorbable scaffolds for the treatment of in-stent restenosis. Heart Vessel. 2015, 30, 265–269. [Google Scholar] [CrossRef]
- Wenaweser, P.; Daemen, J.; Zwahlen, M.; van Domburg, R.; Juni, P.; Vaina, S.; Hellige, G.; Tsuchida, K.; Morger, C.; Boersma, E.; et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice—4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 2008, 52, 1134–1140. [Google Scholar] [CrossRef] [Green Version]
- Beshchasna, N.; Saqib, M.; Kraskiewicz, H.; Wasyluk, L.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Ghizdavet, Z.; Marin, A.; Ficai, A.; et al. Recent Advances in Manufacturing Innovative Stents. Pharmaceutics 2020, 12, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriques, J.P.S.; Elias, J. The first generation ABSORB BVS scaffold; to be or not to be? Neth Heart J. 2017, 25, 416–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Fu, G.; Xu, B.; Zhou, Y.; Su, X.; Liu, H.; Zhang, Z.; Yu, B.; Wang, X.; Han, Y.; et al. Safety and efficacy of the novel sirolimus-eluting bioresorbable scaffold for the treatment of de novo coronary artery disease: One-year results from a prospective patient-level pooled analysis of NeoVas trials. Catheter. Cardiovasc. Interv. 2019, 93 (Suppl. S1), 832–838. [Google Scholar] [CrossRef]
- Lupescu, S.; Munteanu, C.; Sindilar, E.V.; Istrate, B.; Mihai, I.; Oprisan, B.; Pasca, A.S. Long-Term Examination of Degradation and In Vivo Biocompatibility of Some Mg-0.5Ca-xY Alloys in Sprague Dawley Rats. Materials 2022, 15, 5958. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.F.; Heagerty, A.M.; Bing, R.F.; Barnett, D.B. Intravenous infusion of magnesium sulphate after acute myocardial infarction: Effects on arrhythmias and mortality. Int. J. Cardiol. 1986, 12, 175–183. [Google Scholar] [CrossRef]
- Li, Q.T.; Ye, W.B.; Gao, H.; Gao, L.L. Improving the corrosion resistance of ZEK100 magnesium alloy by combining high-pressure torsion technology with hydroxyapatite coating. Mater. Des. 2019, 181, 107933. [Google Scholar] [CrossRef]
- Erbel, R.; di Mario, C.; Bartunek, J.; Bonnier, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Heublein, B.; Horrigan, M.; et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: A prospective, non-randomised multicentre trial. Lancet 2007, 369, 1869–1875. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.J.; Kaiser, C.; et al. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur. Heart J. 2016, 37, 2701–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.H.; Cheon, K.H.; Jo, K.I.; Ahn, J.H.; Kim, H.E.; Jung, H.D.; Jang, T.S. An asymmetric surface coating strategy for improved corrosion resistance and vascular compatibility of magnesium alloy stents. Mater. Des. 2020, 196, 109182. [Google Scholar] [CrossRef]
- Park, J.Y.; Gemmelll, C.H.; Davies, J.E. Platelet interactions with titanium: Modulation of platelet activity by surface topography. Biomaterials 2001, 22, 2671–2682. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, J.; Li, L.L.; Jia, K.; Xia, W.; Deng, C. In vitro and in vivo study of Magnesium containing bioactive glass nanoparticles modified gelatin scaffolds for bone repair. Biomed. Mater. 2022, 17, 025018. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, S.F. Formation of micro-arc oxidation coatings on AZ91HP magnesium alloys. Corros. Sci. 2009, 51, 2820–2825. [Google Scholar] [CrossRef]
- Lee, H.P.; Lin, D.J.; Yeh, M.L. Phenolic Modified Ceramic Coating on Biodegradable Mg Alloy: The Improved Corrosion Resistance and Osteoblast-Like Cell Activity. Materials 2017, 10, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, S.T.; Das, M.; Arockiarajan, A. Biocompatibility and corrosion evaluation of niobium oxide coated AZ31B alloy for biodegradable implants. Colloid Surface B. 2022, 212, 112342. [Google Scholar] [CrossRef] [PubMed]
- Menze, R.; Wittchow, E. In vitro and in vivo evaluation of a novel bioresorbable magnesium scaffold with different surface modifications. J. Biomed. Mater. Res. B 2021, 109, 1292–1302. [Google Scholar] [CrossRef]
- Gambaro, S.; Nascimento, M.L.; Shekargoftar, M.; Ravanbakhsh, S.; Sales, V.; Paternoster, C.; Bartosch, M.; Witte, F.; Mantovani, D. Characterization of a Magnesium Fluoride Conversion Coating on Mg-2Y-1Mn-1Zn Screws for Biomedical Applications. Materials 2022, 15, 8245. [Google Scholar] [CrossRef]
- Erisen, D.E.; Zhang, Y.Q.; Zhang, B.C.; Yang, K.; Chen, S.S.; Wang, X.L. Biosafety and biodegradation studies of AZ31B magnesium alloy carotid artery stent in vitro and in vivo. J. Biomed. Mater. Res. B 2022, 110, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Drynda, A.; Hassel, T.; Hoehn, R.; Perz, A.; Bach, F.W.; Peuster, M. Development and biocompatibility of a novel corrodible fluoride-coated magnesium-calcium alloy with improved degradation kinetics and adequate mechanical properties for cardiovascular applications. J. Biomed. Mater. Res. A 2010, 93, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Zhen, Z.; Liu, J.; Xi, T.F.; Zheng, Y.D.; Guan, S.K.; Zheng, Y.F.; Cheng, Y. Multifunctional MgF2/Polydopamine Coating on Mg Alloy for Vascular Stent Application. J. Mater. Sci. Technol. 2015, 31, 733–743. [Google Scholar] [CrossRef]
- Kang, E.Y.; Choi, B.; Park, W.; Kim, I.H.; Han, D.K. One step bulk modification of poly (L-lactic acid) composites with functional additives to improve mechanical and biological properties for cardiovascular implant applications. Colloid Surf. B 2019, 179, 161–169. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Weir, N.A.; Buchanan, F.J.; Orr, J.F.; Farrar, D.F.; Boyd, A. Processing, annealing and sterilisation of poly-L-lactide. Biomaterials 2004, 25, 3939–3949. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Fernandez, N. Medical Devices with Porous Polymeric Structures. U.S. Patent US20150250627A1[P].21.5., 2015. [Google Scholar]
- Ang, H.Y.; Bulluck, H.; Wong, P.; Venkatraman, S.S.; Huang, Y.Y.; Foin, N. Bioresorbable stents: Current and upcoming bioresorbable technologies. Int. J. Cardiol. 2017, 228, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.P.; Yamamoto, A. In vitro degradation of biodegradable polymer-coated magnesium under cell culture condition. Appl. Surf. Sci. 2012, 258, 6353–6358. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Serruys, P.W.; Chevalier, B.; Sotomi, Y.; Cequier, A.; Carrie, D.; Piek, J.J.; van Boven, A.J.; Dominici, M.; Dudek, D.; McClean, D.; et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): A 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016, 388, 2479–2491. [Google Scholar] [CrossRef]
- Zhu, Y.; Sheng, Y.; Zheng, L.; Qin, L.; Ngai, T. Poly (L-lactic acid) (PLLA) Coatings with Controllable Hierarchical Porous Structures on Magnesium Substrate: An Evaluation of Corrosion Behavior and Cytocompatibility. ACS Appl. Bio Mater. 2019, 2, 3843–3853. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X.; Tan, J.Y.; Wu, W.; Petrini, L.; Zhang, L.; Shi, Y.J.; Cattarinuzzi, E.; Pei, J.; Huang, H.; Ding, W.J.; et al. Modeling and Experimental Studies of Coating Delamination of Biodegradable Magnesium Alloy Cardiovascular Stents. ACS Biomater. Sci. Eng. 2018, 4, 3864–3873. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Zhang, X.; Zhang, L.T.; Xie, W.F. Ultrasonic spray coating polymer and small molecular organic film for organic light-emitting devices. Sci. Rep. 2016, 6, 37042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedair, T.M.; Kang, S.N.; Joung, Y.K. A Promising Approach for Improving the Coating Stability and In Vivo Performance of Biodegrable Polymer-Coated Sirolimus-Eluting Stent. J. Biomed. Nanotechnol. 2016, 12, 2015–2028. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. A 2003, 65, 188–195. [Google Scholar] [CrossRef]
- Tadashi, K.; Hiroaki, T. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials 2006, 27, 2907–2915. [Google Scholar]
- Yan, T.T.; Li, K.X.; Jiang, Z.M.; Miao, X.Y.; Li, P.; Chen, Q.H.; Tan, L.L.; Yang, K. Hemocompatibility of Fluoride Treated AZ31B Magnesium Alloys Used for Intravascular Stents. Rare Met. Mater. Eng. 2022, 51, 469–473. [Google Scholar]
- Lisitsyn, V.M.; Lisitsyna, L.A.; Popov, A.I.; Kotomin, E.A.; Maier, J. Stabilization of primary mobile radiation defects in MgF2 crystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 374, 24–28. [Google Scholar] [CrossRef]
- Lisitsyn, V.M.; Korepanov, V.I.; Yakovlev, V.Y. Evolution of primary radiation defects in ionic crystals. Russ. Phys. J. 1996, 39, 1009–1028. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gao, J.C.; Liu, C.L. Effect of Fluoride Treatment on Corrosion Property of AZ31 Magnesium Alloy in Hank’s Solution. Adv. Mater. Res. 2011, 239–242, 186–190. [Google Scholar] [CrossRef]
- Pan, C.J.; Tang, J.J.; Weng, Y.J.; Wang, J.; Huang, N. Preparation, characterization and anticoagulation of curcumin-eluting controlled biodegradable coating stents. J. Control Release 2006, 116, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Tan, L.L.; Teng, Y.X.; Zhang, B.C.; Yang, K. Study of drug-eluting coating on metal coronary stent. Mater. Sci. Eng. C 2013, 33, 1476–1480. [Google Scholar]
- Wang, P.J.; Ferralis, N.; Conway, C.; Grossman, J.C.; Edelman, E.R. Strain-induced accelerated asymmetric spatial degradation of polymeric vascular scaffolds. Proc. Natl. Acad. Sci. USA 2018, 115, 2640–2645. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolic, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.F.; Wang, J.F.; Sheng, K.; Miao, F.L.; Wang, Y.; Zhang, Y.F.; Hou, R.Q.; Mei, D.; Sun, Y.F.; Zheng, Y.F.; et al. Optimizing structural design on biodegradable magnesium alloy vascular stent for reducing strut thickness and raising radial strength. Mater. Des. 2022, 220, 110843. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Sutera, A.; Botta, L.; Fontana, R.M.; Gallo, G. Plasma modified PLA electrospun membranes for actinorhodin production intensification in Streptomyces coelicolor immobilized-cell cultivations. Colloids Surf B Biointerfaces 2017, 157, 233–241. [Google Scholar] [CrossRef]
- Cipriano, A.F.; Sallee, A.; Tayoba, M.; Alcaraz, M.C.C.; Lin, A.; Guan, R.G.; Zhao, Z.Y.; Liu, H.N. Cytocompatibility and early inflammatory response of human endothelial cells in direct culture with Mg-Zn-Sr alloys. Acta Biomater. 2017, 48, 499–520. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.S.; Tian, Q.M.; Vuong, T.; Shashaty, M.; Gopez, C.; Sanders, T.; Liu, H.N. Comparison Study on Four Biodegradable Polymer Coatings for Controlling Magnesium Degradation and human Endothelial Cell Adhesion and Spreading. ACS Biomater. Sci. Eng. 2017, 3, 936–950. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Najafinezhad, A.; Hamzah, E.; Ismail, A.F.; Berto, F.; Chen, X.B. Clinoenstatite/Tantalum Coating for Enhancement of Biocompatibility and Corrosion Protection of Mg Alloy. J. Funct. Biomater. 2020, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.W.; Rahmati, M.; Barrantes, A.; Haugen, H.J.; Mirtaheri, P. In Vitro Monitoring of Magnesium-Based Implants Degradation by Surface Analysis and Optical Spectroscopy. Int. J. Mol. Sci. 2022, 23, 6099. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.S.; Wang, J.G.; Wang, H.Y.; Liu, Y.; Li, S.Y.; Li, D.W. Anti-corrosion behaviour of superwetting structured surfaces on Mg-9Al-1Zn magnesium alloy. Appl. Surf. Sci. 2019, 483, 1017–1026. [Google Scholar] [CrossRef]
- Weng, Y.J.; Song, Q.A.; Zhou, Y.J.; Zhang, L.P.; Wang, J.; Chen, J.Y.; Leng, Y.X.; Li, S.Y.; Huang, N. Immobilization of selenocystamine on TiO2 surfaces for in situ catalytic generation of nitric oxide and potential application in intravascular stents. Biomaterials 2011, 32, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.N.; Zheng, Y.F.; Cheng, Y.; Zhong, S.P.; Xi, T.F. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Ward, C.A.; Stanga, R.D.; Zingg, W.; Herbert, M.A. Relation of proteins, platelets, and gas nuclei in adhesion to synthetic materials. Am. J. Physiol. 1977, 233, 100–105. [Google Scholar] [CrossRef]














| Sequence | Name | Quantity |
|---|---|---|
| 1 | NaCl | 8.035 g |
| 2 | NaHCO3 | 0.355 g |
| 3 | KCl | 0.225 g |
| 4 | K2HPO4·3H2O | 0.231 g |
| 5 | MgCl2·6H2O | 0.311 g |
| 6 | 1.0 M-HCl | 39 mL |
| 7 | CaCl2 | 0.292 g |
| 8 | Na2SO4 | 0.072 g |
| 9 | Tris | 6.118 g |
| 10 | 1.0 M-HCl | 0–5 mL |
| Samples | Bare Mg | HF-Mg | PLLA (5)-HF-Mg | PLLA (10)-HF-Mg |
|---|---|---|---|---|
| Icorr | 958 ± 5 | 143 ± 3.5 | 81 ± 1.8 | 70 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Chen, Y.; Fan, H.; Chen, S.; Wang, H.; Song, X. A Novel PLLA/MgF2 Coating on Mg Alloy by Ultrasonic Atomization Spraying for Controlling Degradation and Improving Biocompatibility. Materials 2023, 16, 682. https://doi.org/10.3390/ma16020682
Peng W, Chen Y, Fan H, Chen S, Wang H, Song X. A Novel PLLA/MgF2 Coating on Mg Alloy by Ultrasonic Atomization Spraying for Controlling Degradation and Improving Biocompatibility. Materials. 2023; 16(2):682. https://doi.org/10.3390/ma16020682
Chicago/Turabian StylePeng, Wenpeng, Yizhe Chen, Hongde Fan, Shanshan Chen, Hui Wang, and Xiang Song. 2023. "A Novel PLLA/MgF2 Coating on Mg Alloy by Ultrasonic Atomization Spraying for Controlling Degradation and Improving Biocompatibility" Materials 16, no. 2: 682. https://doi.org/10.3390/ma16020682
APA StylePeng, W., Chen, Y., Fan, H., Chen, S., Wang, H., & Song, X. (2023). A Novel PLLA/MgF2 Coating on Mg Alloy by Ultrasonic Atomization Spraying for Controlling Degradation and Improving Biocompatibility. Materials, 16(2), 682. https://doi.org/10.3390/ma16020682