Antitumor Effects of Microencapsulated Gratiola officinalis Extract on Breast Carcinoma and Human Cervical Cancer Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials for Microcapsule Preparation
2.2. Gratiola officinalis L. Extract Preparation and Description of the Chemical Composition
2.3. Polyelectrolyte Microcapsules Preparation Method
2.4. Cultivation of SK-BR-3 and HeLa Cell Lines
2.5. Optical and Fluorescence Microscopy Statistical Analysis
3. Results
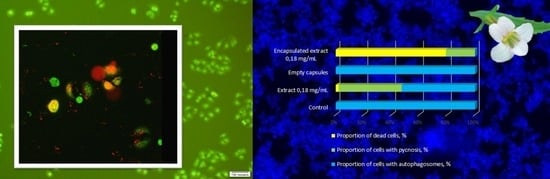
3.1. Effect of the Encapsulated Extract Gratiola officinalis L. on Breast Carcinoma Cells (SK-BR-3)
3.2. Effect of the Encapsulated Extract Gratiola officinalis L. on Human Cervical Cancer (HeLa) Cells
3.3. Morphological Changes after Exposure to Non-Encapsulated Gratiola officinalis Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Cock, L.J.; De Koker, S.; De Geest, B.G.; Grooten, J.; Vervaet, C.; Remon, J.P.; Sukhorukov, G.B.; Antipina, M.N. Polymeric multilayer capsules in drug delivery. Angew. Chem. Int. Ed. Engl. 2010, 49, 6954–6973. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.C.; Javier, D.J.; Richards-Kortum, R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int. J. Cancer 2008, 123, 1979–1990. [Google Scholar] [CrossRef]
- Alencar, H.; Funovics, M.A.; Figueiredo, J.; Sawaya, H.; Weissleder, R.; Mahmood, U. Colonic adenocarcinomas: Near-infrared microcatheter imaging of smart probes for early detection—Study in mice. Radiology 2007, 244, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Kalenichenko, D.; Nifontova, G.; Karaulov, A.; Sukhanova, A.; Nabiev, I. Designing functionalized polyelectrolyte microcapsules for cancer treatment. Nanomaterials 2021, 11, 3055. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.K.; Matey, L. Chemotherapy and Immunotherapy Guidelines and Recommendations for Practice; Oncology Nursing Society: Pittsburgh, PA, USA, 2019; pp. 25–50. [Google Scholar]
- Helleday, T. Chemotherapy-induced toxicity—A secondary effect caused by released DNA? Ann. Oncol. 2017, 28, 2054–2055. [Google Scholar] [CrossRef]
- Gao, B.; Yang, F.; Chen, W.; Li, R.; Hu, X.; Liang, Y.; Li, D. Multidrug resistance affects the prognosis of primary epithelial ovarian cancer. Oncol Lett. 2019, 18, 4262–4269. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Denton, D.; Kumar, S. Hedgehog and Wingless signaling are not essential for autophagy-dependent cell death. Biochem. Pharmacol. 2019, 162, 3–13. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Huang, Y.-C.; Thiyagarajan, V.; Mathew, D.C.; Lin, K.-Y.; Chen, S.-C.; Liu, J.-Y.; Hsu, L.-S.; Li, M.-L.; Yang, H.-L. Anticancer activities of chalcone flavokawain B from Alpinia pricei Hayata in human lung adenocarcinoma (A549) cells via induction of reactive oxygen species-mediated apoptotic and autophagic cell death. J. Cell. Physiol. 2019, 234, 17514–17526. [Google Scholar] [CrossRef]
- Polier, G.; Ding, J.; Konkimalla, B.; Eick, D.; Ribeiro, N.; Kohler, R.; Giaisi, M.; Efferth, T.; Desaubry, L.; Krammer, P.H.; et al. Wogonin and related natural flavones are inhibitors of CDK9 that induce apoptosis in cancer cells by transcriptional suppression of Mcl-1. Cell Death Dis. 2011, 2, e182. [Google Scholar] [CrossRef] [Green Version]
- Isaykina, N.V.; Kalinkina, G.I.; Razina, T.G.; Zueva, E.P.; Rybalkina, O.Y.; Ulrikh, A.V.; Fedorova, E.P.; Shilova, A.B. Fruits of Sorbus aucuparia L. are source drug for increase of tumors effectiveness chemotherapy. Chem. Plant Mater. 2017, 4, 165–173. [Google Scholar] [CrossRef]
- Costea, T.; Hudit, A.; Ciolac, O.A.; Gălăt, B.; Ginghină, O.; Costache, M.; Ganea, C.; Mocanu, M.M. Chemoprevention of colorectal cancer by dietary compounds. Int. J. Mol. Sci. 2018, 19, 3787. [Google Scholar] [CrossRef]
- Koosha, S.; Alshawsh, M.A.; Looi, C.Y.; Seyedan, A.; Mohamed, Z. An association map on the effect of flavonoids on the signaling pathways in colorectal cancer. Int. J. Med. Sci. 2016, 13, 374–385. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Mironescu, A.; Dracea, L.; Ples, L. The role of natural polyphenols in the prevention and treatment of cervical cancer—An overview. Molecules 2016, 21, 1055. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Erratum to: Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 70. [Google Scholar] [CrossRef]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell. Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef]
- Lim, W.; Ryu, S.; Bazer, F.W.; Kim, S.M.; Song, G. Chrysin attenuates progression of ovarian cancer cells by regulating signaling cascades and mitochondrial dysfunction. J. Cell. Physiol. 2018, 233, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Donovan, M.G.; Papoutsis, A.J.; Doetschman, T.C.; Selmin, O.I. Genistein prevents BRCA1 CpG methylation and proliferation in human breast cancer cells with activated aromatic hydrocarbon receptor. Curr. Dev. Nutr. 2017, 1, e000562. [Google Scholar] [CrossRef]
- Granato, M.; GilardiniMontani, M.S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017, 36, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, R.P.; Bonfim-Mendonca, P.D.; Gimenes, F.; Ratti, B.A.; Kaplum, V.; Bruschi, M.L.; Nakamura, C.V.; Silva, S.O.; Maria-Engler, S.S.; Consolarp, M.E. Oxidative stress triggered by apigenin induces apoptosis in a comprehensive panel of human cervical cancer-derived cell lines. Oxid. Med. Cell. Longev. 2017, 2017, 1512745. [Google Scholar] [CrossRef]
- Sung, B.; Chung, H.Y.; Kim, N.D. Role of apigenin in cancer prevention via the induction of apoptosis and autophagy. J. Cancer Prev. 2016, 21, 216–226. [Google Scholar] [CrossRef]
- Ci, Y.; Qiao, J.; Han, M. Molecular mechanisms and metabolomics of natural polyphenols interfering with breast cancer metastasis. Molecules 2016, 21, 1634. [Google Scholar] [CrossRef] [PubMed]
- Borodina, T.N.; Rumsh, L.D.; Kunizhev, S.M.; Sukhorukov, G.B.; Vorozhtsov, G.N.; Feldman, B.M.; Markvicheva, E.A. Polyelectrolyte microcapsules as the systems for delivery of biologically active substances. Biochem. (Mosc.) Suppl. B Biomed. Chem. 2008, 2, 88–93. [Google Scholar] [CrossRef]
- Inozemtseva, O.A.; German, S.V.; Navolokin, N.A.; Bucharskaya, A.B.; Maslyakova, G.N.; Gorin, D.A. Chapter 6—Encapsulated Magnetite Nanoparticles: Preparation and Application as Multifunctional Tool for Drug Delivery Systems. In Advanced Nanomaterials, Nanotechnology and Biosensors; Nikolelis, D.P., Nikoleli, G.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–192. [Google Scholar]
- Demina, P.A.; Abalymov, A.A.; Voronin, D.V.; Sadovnikov, A.V.; Lomova, M.V. Highly-magnetic mineral protein–tannin vehicles with anti-breast cancer activity. Mater. Chem. Front. 2021, 5, 2007–2018. [Google Scholar] [CrossRef]
- Van der Meeren, L.; Li, J.; Parakhonskiy, B.V.; Krysko, D.V.; Skirtach, A.G. Classification of analytics, sensorics, and bioanalytics with polyelectrolyte multilayer capsules. Anal. Bioanal. Chem. 2020, 412, 5015–5029. [Google Scholar] [CrossRef]
- Lomova, M.V.; Brichkina, A.I.; Kiryukhin, M.V.; Vasina, E.N.; Pavlov, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Antipina, M.N. Multilayer Capsules of Bovine Serum Albumin and Tannic Acid for Controlled Release by Enzymatic Degradation. ACS Appl. Mater. Interfaces 2015, 7, 11732–11740. [Google Scholar] [CrossRef]
- Svenskaya, Y.; Garello, F.; Lengert, E.; Kozlova, A.; Verkhovskii, R.; Bitonto, V.; Ruggiero, M.R.; German, S.; Gorin, D.; Terreno, E. Biodegradable polyelectrolyte/magnetite capsules for MR imaging and magnetic targeting of tumors. Nanotheranostics 2021, 5, 362–377. [Google Scholar] [CrossRef]
- Navolokin, N.A.; German, S.V.; Bucharskaya, A.B.; Godage, O.S.; Zuev, V.V.; Maslyakova, G.N.; Pyataev, N.A.; Zamyshliaev, P.S.; Zharkov, M.N.; Terentyuk, G.S.; et al. Systemic administration of polyelectrolyte microcapsules: Where do they accumulate and when? In vivo and ex vivo study. Nanomaterials 2018, 8, 812. [Google Scholar] [CrossRef] [PubMed]
- Polukonova, N.V.; Durnova, N.A.; Kurchatova, M.N.; Navolokin, N.A.; Golikov, A.G. Chemical analysis of the new biological active composition from Gratiola officinalis L. Chem. Plant Raw Mater. 2013, 4, 165–173. [Google Scholar] [CrossRef]
- Polukonova, N.V.; Navolokin, N.A.; Bucharskaya, A.B.; Mudrak, D.A.; Baryshnikova, M.A.; Stepanova, E.V.; Solomko, E.S.; Polukonova, A.V.; Maslyakova, G.N. The apoptotic activity of flavonoid-containing Gratiola officinalis extract in cell cultures of human kidney cancer. Russ. Open Med. J. 2018, 7, e0402. [Google Scholar] [CrossRef]
- Polukonova, N.N.; Navolokin, N.A.; Baryshnikova, M.A.; Maslyakova, G.N.; Bucharskaya, A.B.; Polukonova, A.V. Examining apoptotic activity of Gratiola officinalis L. (Scrophulariaceae) extract on cultured human tumor cell lines. Russ. Open Med. J. 2022, 11, e0415. [Google Scholar] [CrossRef]
- Navolokin, N.A.; Mudrak, D.A.; Bucharskaya, A.B.; Matveeva, O.V.; Tychina, S.A.; Polukonova, N.V.; Maslyakova, G.N. Effect of flavonoid-containing extracts on the growth of transplanted sarcoma 45, peripheral blood and bone marrow condition after oral and intramuscular administration in rats. Russ. Open Med. J. 2017, 6, e0304. [Google Scholar] [CrossRef]
- WFO (2023): Gratiola officinalis L. World Flora Online. Version 02. 2023. Available online: http://www.worldfloraonline.org/taxon/wfo-0000708733 (accessed on 15 December 2022).
- Shirokov, A.; Grinev, V.; Polukonova, N.; Verkhovsky, R.; Doroshenko, A.; Mudrak, D.; Navolokin, N.; Polukonova, A.; Bucharskaya, A.; Maslyakova, G. Isolation, spectral characterization and biological activity of fractions of the flavonoid-containing Gratiola officinalis L. extract. BioRxiv 2020. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Donath, E.; Davis, S.; Lichtenfeld, H.; Caruso, F.; Popov, V.I.; Mohwald, H. Stepwise polyelectrolyte assembly on particle surfaces: A novel approach to colloid design. Polym. Adv. Technol. 1998, 9, 759–767. [Google Scholar] [CrossRef]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein—Calcium carbonate coprecipitation: A tool for protein encapsulation. Biotechnol. Prog. 2005, 21, 918–925. [Google Scholar] [CrossRef]
- Demina, P.A.; Saveleva, M.S.; Anisimov, R.A.; Prikhozhdenko, E.S.; Voronin, D.V.; Abalymov, A.A.; Cherednichenko, K.A.; Timaeva, O.I.; Lomova, M.V. Degradation of hybrid drug delivery carriers with a mineral core and a protein–tannin shell under proteolytic hydrolases. Biomimetics 2022, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Usov, D.; Sukhorukov, G.B. Dextran coatings for aggregation control of layer-by-layer assembled polyelectrolyte microcapsules. Langmuir 2010, 26, 12575–12584. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.L. On the comparison of several mean values: An alternative approach. Biometrika 1951, 38, 330–336. [Google Scholar] [CrossRef]
- Navolokin, N.A.; Polukonova, N.V.; Mudrak, D.A.; Myl’nikov, A.M.; Baryshnikova, M.A.; Khochenkov, D.A.; Bucharskaya, A.B.; Polukonova, A.V.; Maslyakova, G.N. Advantages and possibilities of fluorescence-based methods for the visualization of apoptosis and autophagy in human tumor cells in vitro. Opt. Spectrosc. 2019, 126, 693–702. [Google Scholar] [CrossRef]
- Bora, A.F.M.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Arora, D.; Saneja, A.; Jaglan, S. Cyclodextrin-based delivery systems for dietary pharmaceuticals. Environ. Chem. Lett. 2019, 17, 1263–1270. [Google Scholar] [CrossRef]
- Bilia, A.R.; Isacchi, B.; Righeschi, C.; Guccione, C.; Bergonzi, M.C. Flavonoids loaded in nanocarriers: An opportunity to increase oral bioavailability and bioefficacy. Food Nutr. Sci. 2014, 5, 1212–1327. [Google Scholar] [CrossRef]
- Simão, A.M.S.; Bolean, M.; Cury, T.A.C.; Stabeli, R.G.; Itri, R.; Ciancaglini, P. Liposomal systems as carriers for bioactive compounds. Biophys. Rev. 2015, 7, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Kharanaghi, E.A.; Arough, J.M.; Zandieh-Doulabi, B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. Int. J. Polym. Mater. 2018, 67, 383–400. [Google Scholar] [CrossRef]
- Steiner, B.M.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci. Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Navolokin, N.A.; Maslyakova, G.N.; Polukonova, N.V.; Mudrak, D.A.; Bucharskaya, A.B. The expression of apoptosis and autophagy markers in transplantable rat kidney cancer with the administration of flavonoid-containing hedge hyssop (L.) extract. Arch. Pathol. 2019, 81, 24–30. [Google Scholar] [CrossRef]
- Mylnikov, A.M.; Polukonova, N.V.; Isaev, D.S.; Doroshenko, A.A.; Verkhovsky, R.A.; Nikolaeva, N.A.; Mudrak, D.A.; Navolokin, N.A. Identification of the death pathways of human kidney carcinoma A498cells under the action of Gratiola officinalis extract and green tea flavonoids using fluorescent imaging methods. Opt. Spectrosc. 2020, 128, 964–971. [Google Scholar] [CrossRef]







| Cellculture | SK-BR-3 | HeLa | ||||
|---|---|---|---|---|---|---|
| Groups | Number of Viable Cells | Proportion of Cells in Apoptosis | Number of Viable Cells | Proportion of Dead Cells, % | Proportion of Cells with Pycnosis, % | Proportion of Cells with Autophagosomes, % |
| Control | 365 ± 24.59 | 0.56 ± 0.3 | 324 ± 32.3 | 0 ± 0 | 0 ± 0 | 1 ± 1 |
| Extract 0.18 mg/mL | 354.6 ± 4.2 | 2.3 ± 0.04 * | 168 ± 15 * | 2.8 ± 0.5 * | 61 ± 8 * | 71 ± 11 * |
| Extract 0.9 mg/mL | 236.6 ± 7.8 * | 86.3 ± 1.4 * | 133 ± 12 * | 13.2 ± 4 * | 71 ± 11 * | 0.3 ± 0.1 * |
| Empty capsules | 342 ± 11 | 1 ± 0.5 | 330 ± 40.8 | 0 ± 0 | 0 ± 0 | 54 ± 10 * |
| Encapsulated extract 0.18 mg/mL | 0 ± 0 * | 0 ± 0 * | 180 ± 19 * | 34 ± 0.5 * | 9 ± 3 * | 0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navolokin, N.; Lomova, M.; Bucharskaya, A.; Godage, O.; Polukonova, N.; Shirokov, A.; Grinev, V.; Maslyakova, G. Antitumor Effects of Microencapsulated Gratiola officinalis Extract on Breast Carcinoma and Human Cervical Cancer Cells In Vitro. Materials 2023, 16, 1470. https://doi.org/10.3390/ma16041470
Navolokin N, Lomova M, Bucharskaya A, Godage O, Polukonova N, Shirokov A, Grinev V, Maslyakova G. Antitumor Effects of Microencapsulated Gratiola officinalis Extract on Breast Carcinoma and Human Cervical Cancer Cells In Vitro. Materials. 2023; 16(4):1470. https://doi.org/10.3390/ma16041470
Chicago/Turabian StyleNavolokin, Nikita, Maria Lomova, Alla Bucharskaya, Olga Godage, Natalya Polukonova, Alexander Shirokov, Vyacheslav Grinev, and Galina Maslyakova. 2023. "Antitumor Effects of Microencapsulated Gratiola officinalis Extract on Breast Carcinoma and Human Cervical Cancer Cells In Vitro" Materials 16, no. 4: 1470. https://doi.org/10.3390/ma16041470
APA StyleNavolokin, N., Lomova, M., Bucharskaya, A., Godage, O., Polukonova, N., Shirokov, A., Grinev, V., & Maslyakova, G. (2023). Antitumor Effects of Microencapsulated Gratiola officinalis Extract on Breast Carcinoma and Human Cervical Cancer Cells In Vitro. Materials, 16(4), 1470. https://doi.org/10.3390/ma16041470