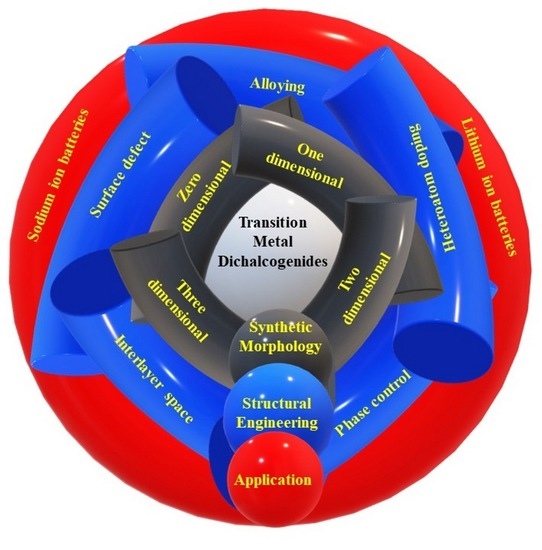
Recent Advancement and Structural Engineering in Transition Metal Dichalcogenides for Alkali Metal Ions Batteries
Abstract
:1. Introduction
2. Preparative Strategies for L-TMDs Based Composites
2.1. 0D (NPs) Based L-TMDs Composites
2.2. 1D Based L-TMDs Composites
2.3. 2D Based L-TMDs Composites
2.4. 3D (2D Graphene) Based L-TMDs Composites
2.5. 3D (Bulk Materials) Based L-TMDs Composites
2.6. Non-Crytalline Carbon Based L-TMDs Composites
3. Structural Engineering in L-TMDs
3.1. Interlayer Spacing Engineering
3.2. Surface Defect Engineering
3.3. Phase Controlling Engineering
3.4. Doping Engineering
3.5. Alloy Engineering
4. Prospective Application of L-TMDs Based Composite
4.1. Lithium-Ion Batteries (LIBs)
4.2. Sodium Ion Batteries (SIBs)
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vesborg, P.C.; Jaramillo, T.F. Addressing the terawatt challenge: Scalability in the supply of chemical elements for renewable energy. Rsc. Adv. 2012, 2, 7933–7947. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, N.; Hou, Y. Electrode nanostructures in lithium-based batteries. Adv. Sci. 2014, 1, 1400012. [Google Scholar] [CrossRef] [PubMed]
- Croguennec, L.; Palacin, M.R. Recent achievements on inorganic electrode materials for lithium-ion batteries. J. Am. Chem. Soc. 2015, 137, 3140–3156. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.V.; Subba Rao, G.; Chowdari, B. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Palacin, M.R. Recent advances in rechargeable battery materials: A chemist’s perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Zhamu, A.; Chen, G.; Liu, C.; Neff, D.; Fang, Q.; Yu, Z.; Xiong, W.; Wang, Y.; Wang, X.; Jang, B.Z. Reviving rechargeable lithium metal batteries: Enabling next-generation high-energy and high-power cells. Energy Environ. Sci. 2012, 5, 5701–5707. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on advanced materials for Li-ion batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Brutti, S.; Gentili, V.; Menard, H.; Scrosati, B.; Bruce, P.G. TiO2-(B) nanotubes as anodes for lithium batteries: Origin and mitigation of irreversible capacity. Adv. Energy Mater. 2012, 2, 322–327. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Charest, P.; Rodrigues, I.; Mauger, A.; Julien, C.M. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sources 2011, 196, 3949–3954. [Google Scholar] [CrossRef]
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Baker, J. New technology and possible advances in energy storage. Energy Policy 2008, 36, 4368–4373. [Google Scholar] [CrossRef]
- Chalk, S.G.; Miller, J.F. Key challenges and recent progress in batteries, fuel cells, and hydrogen storage for clean energy systems. J. Power Sources 2006, 159, 73–80. [Google Scholar] [CrossRef]
- Yaksic, A.; Tilton, J.E. Using the cumulative availability curve to assess the threat of mineral depletion: The case of lithium. Resources 2009, 34, 185–194. [Google Scholar] [CrossRef]
- Meister, P.; Jia, H.; Li, J.; Kloepsch, R.; Winter, M.; Placke, T. Best practice: Performance and cost evaluation of lithium ion battery active materials with special emphasis on energy efficiency. Chem. Mater. 2016, 28, 7203–7217. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Pramudita, J.C.; Sehrawat, D.; Goonetilleke, D.; Sharma, N. An initial review of the status of electrode materials for potassium-ion batteries. Adv. Energy Mater. 2017, 7, 1602911. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem. Int. Ed. 2015, 54, 3431–3448. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L. Na-ion battery anodes: Materials and electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Liu, X.; Mei, Y.; Huang, Y. Nanostructured Mo-based electrode materials for electrochemical energy storage. Chem. Soc. Rev. 2015, 44, 2376–2404. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, Y.; Chen, D.; Ruoff, R.S.; Yu, G. Two-dimensional materials for beyond-lithium-ion batteries. Adv. Energy Mater. 2016, 6, 1600025. [Google Scholar] [CrossRef]
- Mei, Y.; Huang, Y.; Hu, X. Nanostructured Ti-based anode materials for Na-ion batteries. J. Mater. Chem. A 2016, 4, 12001–12013. [Google Scholar] [CrossRef]
- Zeng, L.; Luo, F.; Chen, X.; Xu, L.; Xiong, P.; Feng, X.; Luo, Y.; Chen, Q.; Wei, M.; Qian, Q. An ultra-small few-layer MoS2-hierarchical porous carbon fiber composite obtained via nanocasting synthesis for sodium-ion battery anodes with excellent long-term cycling performance. Dalton Trans. 2019, 48, 4149–4156. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chao, D.; Liu, E.; Jaroniec, M.; Zhao, N.; Qiao, S.-Z. Transition metal dichalcogenides for alkali metal ion batteries: Engineering strategies at the atomic level. Energy Environ. Sci. 2020, 13, 1096–1131. [Google Scholar] [CrossRef]
- Srivastava, M.; Singh, J.; Kuila, T.; Layek, R.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene and its metal-oxide hybrid nanostructures for lithium-ion batteries. Nanoscale 2015, 7, 4820–4868. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zeng, Z.; Fan, Z.; Liu, J.; Zhang, H. Graphene-based electrodes. Adv. Mater. 2012, 24, 5979–6004. [Google Scholar] [CrossRef] [PubMed]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Savjani, N.; Lewis, E.A.; Bissett, M.A.; Brent, J.R.; Dryfe, R.A.; Haigh, S.J.; O’Brien, P. Synthesis of lateral size-controlled monolayer 1 H-MoS2@oleylamine as supercapacitor electrodes. Chem. Mater. 2016, 28, 657–664. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, Z.; Cabrera, C.R.; Chen, Z. Graphene, inorganic graphene analogs and their composites for lithium ion batteries. J. Mater. Chem. A 2014, 2, 12104–12122. [Google Scholar] [CrossRef]
- Pumera, M.; Sofer, Z.; Ambrosi, A. Layered transition metal dichalcogenides for electrochemical energy generation and storage. J.Mater. Chem. A 2014, 2, 8981–8987. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef]
- Chen, D.; Chen, W.; Ma, L.; Ji, G.; Chang, K.; Lee, J.Y. Graphene-like layered metal dichalcogenide/graphene composites: Synthesis and applications in energy storage and conversion. Mater. Today 2014, 17, 184–193. [Google Scholar] [CrossRef]
- Rao, C.; Nag, A. Inorganic analogues of graphene. Eur. J. Inorg. Chem. 2010, 2010, 4244–4250. [Google Scholar] [CrossRef]
- Kang, W.; Wang, Y.; Xu, J. Recent progress in layered metal dichalcogenide nanostructures as electrodes for high-performance sodium-ion batteries. J. Mater. Chem. A 2017, 5, 7667–7690. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, H.; Hong, S.S.; Li, Y.; Cui, Y. Physical and chemical tuning of two-dimensional transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2664–2680. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.; Zhang, W.; Lee, C.S. Interlayer nanoarchitectonics of two-dimensional transition-metal dichalcogenides nanosheets for energy storage and conversion applications. Adv. Energy Mater. 2017, 7, 1700571. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Ko, Y.N.; Choi, S.; Park, S.; Kang, Y. Hierarchical MoSe2 yolk–shell microspheres with superior Na-ion storage properties. Nanoscale 2014, 6, 10511–10515. [Google Scholar] [CrossRef]
- André, B.; Gustavsson, F.; Svahn, F.; Jacobson, S. Performance and tribofilm formation of a low-friction coating incorporating inorganic fullerene like nano-particles. Surf. Coat. Technol. 2012, 206, 2325–2329. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, H.; Cho, J. MoS2 nanoplates consisting of disordered graphene-like layers for high rate lithium battery anode materials. Nano Lett. 2011, 11, 4826–4830. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Zhang, H. Metal dichalcogenide nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2013, 42, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Whittingham, M.S. Chemistry of intercalation compounds: Metal guests in chalcogenide hosts. Prog. Solid. State Chem. 1978, 12, 41–99. [Google Scholar] [CrossRef]
- Lin, L.; Lei, W.; Zhang, S.; Liu, Y.; Wallace, G.G.; Chen, J. Two-dimensional transition metal dichalcogenides in supercapacitors and secondary batteries. Energy Stor. Mater. 2019, 19, 408–423. [Google Scholar] [CrossRef]
- Chen, B.; Meng, Y.; Sha, J.; Zhong, C.; Hu, W.; Zhao, N. Preparation of MoS2/TiO2 based nanocomposites for photocatalysis and rechargeable batteries: Progress, challenges, and perspective. Nanoscale 2018, 10, 34–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, S.; Pang, H.; Xue, H.; Yu, Y. MoS2-based nanocomposites for electrochemical energy storage. Adv. Sci. 2017, 4, 1600289. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Energy Environ. Science 2014, 7, 209–231. [Google Scholar]
- Wang, H.; Lan, X.; Jiang, D.; Zhang, Y.; Zhong, H.; Zhang, Z.; Jiang, Y. Sodium storage and transport properties in pyrolysis synthesized MoSe2 nanoplates for high performance sodium-ion batteries. J. Power Sources 2015, 283, 187–194. [Google Scholar] [CrossRef]
- Yousaf, M.; Mahmood, A.; Wang, Y.; Chen, Y.; Ma, Z.; Han, R.P. Advancement in layered transition metal dichalcogenide composites for lithium and sodium ion batteries. J. Electr. Eng 2016, 4, 58–74. [Google Scholar]
- Huang, X.; Tan, C.; Yin, Z.; Zhang, H. 25th Anniversary article: Hybrid nanostructures based on two-dimensional nanomaterials. Adv. Mater. 2014, 26, 2185–2204. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhan, J.; Tang, L.; Zhang, Y.; Wang, Y. Recent development of metallic (1T) phase of molybdenum disulfide for energy conversion and storage. Adv. Energy Mater. 2018, 8, 1703482. [Google Scholar] [CrossRef]
- Li, H.; Jia, X.; Zhang, Q.; Wang, X. Metallic transition-metal dichalcogenide nanocatalysts for energy conversion. Chem 2018, 4, 1510–1537. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Hafez, A.M.; Cao, D.; Mukhopadhyay, A.; Ma, Y.; Zhu, H. Metallic MoS2 for high performance energy storage and energy conversion. Small 2018, 14, 1800640. [Google Scholar] [CrossRef]
- Zhu, C.R.; Gao, D.; Ding, J.; Chao, D.; Wang, J. TMD-based highly efficient electrocatalysts developed by combined computational and experimental approaches. Chem. Soc. Rev. 2018, 47, 4332–4356. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Pan, Q.-C.; Yang, G.-H.; Lin, X.-L.; Yan, Z.-X.; Wang, H.-Q.; Huang, Y.-G. Synthesis of Sn/MoS2/C composites as high-performance anodes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 20375–20381. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, Y.; Ren, D.; Lin, S.; Jiang, H.; Li, C. Reciprocal hybridization of MoO2 nanoparticles and few-layer MoS2 for stable lithium-ion batteries. Chem. Commun. 2015, 51, 13838–13841. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, J.; Wen, S.; Lu, L.; Xue, J. Synthesis of SnO2/MoS2 composites with different component ratios and their applications as lithium ion battery anodes. J. Mater. Chem. A 2014, 2, 17857–17866. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Tang, X.; Lu, L.; Xue, J. Ultrasmall Fe3O4 nanoparticle/MoS2 nanosheet composites with superior performances for lithium ion batteries. Small 2014, 10, 1536–1543. [Google Scholar] [CrossRef]
- Pan, L.; Zhu, X.-D.; Xie, X.-M.; Liu, Y.-T. Delicate ternary heterostructures achieved by hierarchical co-assembly of Ag and Fe3O4 nanoparticles on MoS2 nanosheets: Morphological and compositional synergy in reversible lithium storage. J. Mater. Chem. A 2015, 3, 2726–2733. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.T.; Xie, X.M.; Zhu, X.D. Coordination-driven hierarchical assembly of silver nanoparticles on mos2 nanosheets for improved lithium storage. Chem. Asian J. 2014, 9, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Byun, S.; Smith, A.J.; Yu, J.; Huang, J. Enhanced electrocatalytic properties of transition-metal dichalcogenides sheets by spontaneous gold nanoparticle decoration. J. Phys. Chem. Lett. 2013, 4, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zeng, Z.; Bao, S.; Wang, M.; Qi, X.; Fan, Z.; Zhang, H. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat. Commun. 2013, 4, 1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahar, C.; Levi, R.; Cohen, S.R.; Tenne, R. Gold nanoparticles as surface defect probes for WS2 nanostructures. J. Phys. Chem. lett. 2010, 1, 540–543. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Fu, Y.; Du, K. Ultrathin molybdenum diselenide nanosheets anchored on multi-walled carbon nanotubes as anode composites for high performance sodium-ion batteries. J. Power Sources 2015, 296, 2–9. [Google Scholar] [CrossRef]
- Zhai, C.; Du, N.; Zhang, H.; Yu, J.; Yang, D. Multiwalled carbon nanotubes anchored with SnS2 nanosheets as high-performance anode materials of lithium-ion batteries. ACS Appl. Mater. Interfaces 2011, 3, 4067–4074. [Google Scholar] [CrossRef]
- Park, S.-K.; Yu, S.-H.; Woo, S.; Quan, B.; Lee, D.-C.; Kim, M.K.; Sung, Y.-E.; Piao, Y. A simple L-cysteine-assisted method for the growth of MoS2 nanosheets on carbon nanotubes for high-performance lithium ion batteries. Dalton Trans. 2013, 42, 2399–2405. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Lou, X.W. Glucose-assisted growth of MoS2 nanosheets on CNT backbone for improved lithium storage properties. Chem. Eur. J. 2011, 17, 13142–13145. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. L-cysteine-assisted synthesis of layered MoS2/graphene composites with excellent electrochemical performances for lithium ion batteries. ACS Nano 2011, 5, 4720–4728. [Google Scholar] [CrossRef]
- Kong, D.; He, H.; Song, Q.; Wang, B.; Lv, W.; Yang, Q.-H.; Zhi, L. Rational design of MoS2@graphene nanocables: Towards high performance electrode materials for lithium ion batteries. Energy Environ. Sci. 2014, 7, 3320–3325. [Google Scholar] [CrossRef]
- Zhu, C.; Mu, X.; van Aken, P.A.; Yu, Y.; Maier, J. Single-layered ultrasmall nanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium storage. Angew. Chem. 2014, 126, 2184–2188. [Google Scholar] [CrossRef]
- Yu, S.; Jung, J.-W.; Kim, I.-D. Single layers of WS2 nanoplates embedded in nitrogen-doped carbon nanofibers as anode materials for lithium-ion batteries. Nanoscale 2015, 7, 11945–11950. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fan, Z.; Ding, S.; Yu, D.; Du, Y. Fabrication of MoS2 nanosheet@ TiO2 nanotube hybrid nanostructures for lithium storage. Nanoscale 2014, 6, 5245–5250. [Google Scholar] [CrossRef] [Green Version]
- Mao, M.; Mei, L.; Guo, D.; Wu, L.; Zhang, D.; Li, Q.; Wang, T. High electrochemical performance based on the TiO2 nanobelt@ few-layered MoS2 structure for lithium-ion batteries. Nanoscale 2014, 6, 12350–12353. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Li, M.; Cui, P.; Chen, D.; Gengenbach, T.; Chu, L.; Liu, H.; Song, G. Glucose-assisted synthesis of the hierarchical TiO2 nanowire@MoS2 nanosheet nanocomposite and its synergistic lithium storage performance. J. Mater. Chem. A 2015, 3, 2762–2769. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Yang, X.; Qu, Y.; Zhang, Z. Hierarchical MoSe2 nanosheets/reduced graphene oxide composites as anodes for lithium-ion and sodium-ion batteries with enhanced electrochemical performance. ChemNanoMat 2015, 1, 409–414. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, X.; Xu, L.; Xu, X.; Zhang, L.; Chen, W. Ultrathin few-layered molybdenum selenide/graphene hybrid with superior electrochemical Li-storage performance. J. Power Sources 2015, 285, 274–280. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. Single-layer MoS2/graphene dispersed in amorphous carbon: Towards high electrochemical performances in rechargeable lithium ion batteries. J. Mater. Chem. 2011, 21, 17175–17184. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Chen, W.; Chang, K.; Ma, L.; Huang, G.; Chen, D.; Lee, J.Y. CTAB-assisted synthesis of single-layer MoS2–graphene composites as anode materials of Li-ion batteries. J. Mater. Chem. A 2013, 1, 2202–2210. [Google Scholar] [CrossRef]
- Huang, G.; Chen, T.; Chen, W.; Wang, Z.; Chang, K.; Ma, L.; Huang, F.; Chen, D.; Lee, J.Y. Graphene-like MoS2/Graphene composites: Cationic surfactant-assisted hydrothermal synthesis and electrochemical reversible storage of lithium. Small 2013, 9, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, L.; Chen, W.; Huang, G.; Chen, D.; Wang, L.; Lee, J.Y. Facile synthesis of MoS2/graphene composites: Effects of different cationic surfactants on microstructures and electrochemical properties of reversible lithium storage. RSC Adv. 2013, 3, 21675–21684. [Google Scholar] [CrossRef]
- Chang, K.; Geng, D.; Li, X.; Yang, J.; Tang, Y.; Cai, M.; Li, R.; Sun, X. Ultrathin MoS2/nitrogen-doped graphene nanosheets with highly reversible lithium storage. Adv. Energy Mater. 2013, 3, 839–844. [Google Scholar] [CrossRef]
- Sahu, T.S.; Mitra, S. Exfoliated MoS2 sheets and reduced graphene oxide-an excellent and fast anode for sodium-ion battery. Sci. Rep. 2015, 5, 12571. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Ortiz-Quiles, E.O.; Cabrera, C.R.; Chen, Z.; Zhou, Z. Layer-by-layer hybrids of MoS2 and reduced graphene oxide for lithium ion batteries. Electrochim. Acta 2014, 147, 392–400. [Google Scholar] [CrossRef]
- Qu, B.; Ma, C.; Ji, G.; Xu, C.; Xu, J.; Meng, Y.S.; Wang, T.; Lee, J.Y. Layered SnS2-reduced graphene oxide composite–A high-capacity, high-rate, and long-cycle life sodium-ion battery anode material. Adv. Mater. 2014, 26, 3854–3859. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, L.; Wu, Y.; Wang, L.; Yu, Y.; Zhang, X.; Zhao, F. One-step hydrothermal synthesis of SnS2/graphene composites as anode material for highly efficient rechargeable lithium ion batteries. RSC Adv. 2012, 2, 5084–5087. [Google Scholar] [CrossRef]
- Choi, J.; Jin, J.; Jung, I.G.; Kim, J.M.; Kim, H.J.; Son, S.U. SnSe2 nanoplate–graphene composites as anode materials for lithium ion batteries. Chem. Commun. 2011, 47, 5241–5243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, C.; Du, G.; Zhong, Y.; Jiang, J. In situ synthesis of SnS2@graphene nanocomposites for rechargeable lithium batteries. J. Mater. Chem. 2012, 22, 9494–9496. [Google Scholar] [CrossRef]
- Chang, K.; Wang, Z.; Huang, G.; Li, H.; Chen, W.; Lee, J.Y. Few-layer SnS2/graphene hybrid with exceptional electrochemical performance as lithium-ion battery anode. J. Power Sources 2012, 201, 259–266. [Google Scholar] [CrossRef]
- Chen, P.; Su, Y.; Liu, H.; Wang, Y. Interconnected tin disulfide nanosheets grown on graphene for Li-ion storage and photocatalytic applications. ACS Appl. Mater. Interfaces 2013, 5, 12073–12082. [Google Scholar] [CrossRef]
- Luo, B.; Fang, Y.; Wang, B.; Zhou, J.; Song, H.; Zhi, L. Two dimensional graphene–SnS2 hybrids with superior rate capability for lithium ion storage. Energy Environ. Sci. 2012, 5, 5226–5230. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Fu, H.; Guo, B.; Lei, X.; Zhu, Z. Multi-slice nanostructured WS2@ rGO with enhanced Li-ion battery performance and a comprehensive mechanistic investigation. Phys. Chem. Chem. Phys. 2015, 17, 29824–29833. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ji, G.; Ding, B.; Ma, Y.; Qu, B.; Chen, W.; Lee, J.Y. In situ nitrogenated graphene–few-layer WS2 composites for fast and reversible Li+ storage. Nanoscale 2013, 5, 7890–7896. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Rout, C.S.; Yang, J.; Cao, R.; Oh, P.; Shin, H.S.; Cho, J. Freeze-dried WS2 composites with low content of graphene as high-rate lithium storage materials. J. Mater. Chem. A 2013, 1, 14548–14554. [Google Scholar] [CrossRef] [Green Version]
- Shiva, K.; Matte, H.R.; Rajendra, H.; Bhattacharyya, A.J.; Rao, C. Employing synergistic interactions between few-layer WS2 and reduced graphene oxide to improve lithium storage, cyclability and rate capability of Li-ion batteries. Nano Energy 2013, 2, 787–793. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, S.; Liu, Z.; Ma, L.; Vajtai, R.; Ajayan, P.M. Graphene-network-backboned architectures for high-performance lithium storage. Adv. Mater. 2013, 25, 3979–3984. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Huang, Y.; Zhang, L.; Fan, W.; Lai, F.; Liu, T. Electrospun porous carbon nanofiber@ MoS2 core/sheath fiber membranes as highly flexible and binder-free anodes for lithium-ion batteries. Nanoscale 2015, 7, 11093–11101. [Google Scholar] [CrossRef] [Green Version]
- Lingappan, N.; Van, N.H.; Lee, S.; Kang, D.J. Growth of three dimensional flower-like molybdenum disulfide hierarchical structures on graphene/carbon nanotube network: An advanced heterostructure for energy storage devices. J. Power Sources 2015, 280, 39–46. [Google Scholar] [CrossRef]
- Yao, J.; Liu, B.; Ozden, S.; Wu, J.; Yang, S.; Rodrigues, M.-T.F.; Kalaga, K.; Dong, P.; Xiao, P.; Zhang, Y. 3D nanostructured molybdenum diselenide/graphene foam as anodes for long-cycle life lithium-ion batteries. Electrochim. Acta 2015, 176, 103–111. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, S.; Zhan, L.; Ma, L.; Vajtai, R.; Ajayan, P.M. A bottom-up approach to build 3D architectures from nanosheets for superior lithium storage. Adv. Funct. Mater. 2014, 24, 125–130. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, X.; Zhu, Y.; Shen, J.; Fan, K.; Li, C. In situ assembly of graphene sheets-supported SnS2 nanoplates into 3D macroporous aerogels for high-performance lithium ion batteries. J. Power Sources 2013, 237, 178–186. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. WS2@graphene nanocomposites as anode materials for Na-ion batteries with enhanced electrochemical performances. Chem. Commun. 2014, 50, 4192–4195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Wang, Y.; Peng, X. Homogeneously assembling like-charged WS2 and GO nanosheets lamellar composite films by filtration for highly efficient lithium ion batteries. Nano Energy 2014, 7, 25–32. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, J.; Han, S.; Lv, L.; Zhang, F.; Feng, X. Amphiphilic polymer promoted assembly of macroporous Graphene/SnO2 frameworks with tunable porosity for high-performance lithium storage. Small 2014, 10, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Qi, X.; Han, W.; Ren, L.; Liu, Y.; Wang, X.; Zhong, J. SnS2 nanoplates embedded in 3D interconnected graphene network as anode material with superior lithium storage performance. Appl. Surf. Sci. 2015, 355, 7–13. [Google Scholar] [CrossRef]
- Huang, G.; Liu, H.; Wang, S.; Yang, X.; Liu, B.; Chen, H.; Xu, M. Hierarchical architecture of WS2 nanosheets on graphene frameworks with enhanced electrochemical properties for lithium storage and hydrogen evolution. J. Mater. Chem. A 2015, 3, 24128–24138. [Google Scholar] [CrossRef]
- Guo, B.; Yu, K.; Fu, H.; Hua, Q.; Qi, R.; Li, H.; Song, H.; Guo, S.; Zhu, Z. Firework-shaped TiO₂ microspheres embedded with few-layer MoS₂ as an anode material for excellent performance lithium-ion batteries. J. Mater. Chem. A 2015, 3, 6392–6401. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Cao, X.; Yin, Z.; Zhang, H. Three-dimensional graphene materials: Preparation, structures and application in supercapacitors. Energy Environ. Sci. 2014, 7, 1850–1865. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Shi, Y.; Shi, W.; Rui, X.; Yan, Q.; Kong, J.; Zhang, H. Preparation of MoS2-coated three-dimensional graphene networks for high-performance anode material in lithium-ion batteries. Small 2013, 9, 3433–3438. [Google Scholar] [CrossRef]
- Wang, C.; Wan, W.; Huang, Y.; Chen, J.; Zhou, H.H.; Zhang, X.X. Hierarchical MoS2 nanosheet/active carbon fiber cloth as a binder-free and free-standing anode for lithium-ion batteries. Nanoscale 2014, 6, 5351–5358. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Mallavajula, R.; Jayaprakash, N.; Archer, L.A. Self-assembled MoS2–carbon nanostructures: Influence of nanostructuring and carbon on lithium battery performance. J. Mater. Chem. 2012, 22, 12988–12992. [Google Scholar] [CrossRef]
- Hu, S.; Chen, W.; Zhou, J.; Yin, F.; Uchaker, E.; Zhang, Q.; Cao, G. Preparation of carbon coated MoS2 flower-like nanostructure with self-assembled nanosheets as high-performance lithium-ion battery anodes. J. Mater. Chem. A 2014, 2, 7862–7872. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Seng, K.H.; Chou, S.-L.; Wang, J.-Z.; Guo, Z.; Wexler, D.; Liu, H.-K.; Dou, S.-X. Reversible sodium storage via conversion reaction of a MoS2–C composite. Chem. Commun. 2014, 50, 10730–10733. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W.; Ma, L.; Li, H.; Li, H.; Huang, F.; Xu, Z.; Zhang, Q.; Lee, J.-Y. Graphene-like MoS2/amorphous carbon composites with high capacity and excellent stability as anode materials for lithium ion batteries. J. Mater. Chem. 2011, 21, 6251–6257. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Chen, D. Sheet-like MoSe2/C composites with enhanced Li-ion storage properties. J. Mater. Chem. A 2015, 3, 11857–11862. [Google Scholar] [CrossRef]
- Yu, X.Y.; Hu, H.; Wang, Y.; Chen, H.; Lou, X.W. Ultrathin MoS2 nanosheets supported on N-doped carbon nanoboxes with enhanced lithium storage and electrocatalytic properties. Angew. Chem. 2015, 127, 7503–7506. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Fu, Y.; Li, Q. Porous hollow carbon spheres decorated with molybdenum diselenide nanosheets as anodes for highly reversible lithium and sodium storage. Nanoscale 2015, 7, 10198–10203. [Google Scholar] [CrossRef]
- Tongay, S.; Sahin, H.; Ko, C.; Luce, A.; Fan, W.; Liu, K.; Zhou, J.; Huang, Y.-S.; Ho, C.-H.; Yan, J. Monolayer behaviour in bulk ReS2 due to electronic and vibrational decoupling. Nat. Commun. 2014, 5, 3252. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Tan, S.; Mendes, R.G.; Sun, Z.; Chen, Y.; Kong, X.; Xue, Y.; Rümmeli, M.H.; Wu, X.; Chen, S. Extremely weak van der waals coupling in vertical ReS2 nanowalls for high-current-density lithium-ion batteries. Adv. Mater. 2016, 28, 2616–2623. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Qiu, B.; Zhou, S.; Zhang, M.; Huang, H.; Wang, B.; Zhao, J.; Sun, X.; Qiu, J. Ultrahigh rate and long-life sodium-ion batteries enabled by engineered surface and near-surface reactions. Adv. Mater. 2018, 30, 1702486. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, D.; Zhou, Z.; Cabrera, C.R.; Chen, Z. Enhanced Li adsorption and diffusion on MoS2 zigzag nanoribbons by edge effects: A computational study. J. Phys. Chem. Lett. 2012, 3, 2221–2227. [Google Scholar] [CrossRef]
- Xu, Y.; Bahmani, F.; Zhou, M.; Li, Y.; Zhang, C.; Liang, F.; Kazemi, S.H.; Kaiser, U.; Meng, G.; Lei, Y. Enhancing potassium-ion battery performance by defect and interlayer engineering. Nanoscale Horiz. 2019, 4, 202–207. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, X.; Yin, J.; Peng, G.; Cui, P.; Xu, X. MoS2 nanoflowers consisting of nanosheets with a controllable interlayer distance as high-performance lithium ion battery anodes. RSC Adv. 2015, 5, 7938–7943. [Google Scholar] [CrossRef]
- Jing, L.; Lian, G.; Niu, F.; Yang, J.; Wang, Q.; Cui, D.; Wong, C.-P.; Liu, X. Few-atomic-layered hollow nanospheres constructed from alternate intercalation of carbon and MoS2 monolayers for sodium and lithium storage. Nano Energy 2018, 51, 546–555. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Hernandez, F.C.R.; Yoo, H.D.; An, Q.; Yao, Y. Enhancing sodium-ion battery performance with interlayer-expanded MoS2–PEO nanocomposites. Nano Energy 2015, 15, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chen, F.; Tronganh, N.; Lu, M.; Jiang, Y.; Gao, Y.; Jiao, Z.; Cheng, L.; Zhao, B. MoS2/graphene nanocomposite with enlarged interlayer distance as a high performance anode material for lithium-ion battery. J. Mater. Res. 2016, 31, 3151–3160. [Google Scholar] [CrossRef]
- Jiang, H.; Ren, D.; Wang, H.; Hu, Y.; Guo, S.; Yuan, H.; Hu, P.; Zhang, L.; Li, C. 2D monolayer MoS2–carbon interoverlapped superstructure: Engineering ideal atomic interface for lithium ion storage. Adv. Mater. 2015, 27, 3687–3695. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Fu, Y.Q. Defect-mediated lithium adsorption and diffusion on monolayer molybdenum disulfide. Sci. Rep. 2015, 5, 3252. [Google Scholar] [CrossRef] [Green Version]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Ai, K.; Ruan, C.; Shen, M.; Lu, L. MoS2 nanosheets with widened interlayer spacing for high-efficiency removal of mercury in aquatic systems. Adv. Funct. Mater. 2016, 26, 5542–5549. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Chao, D.; Liang, P.; Chen, Z.; Bai, L.; Shen, H.; Liu, X.; Xia, X.; Zhao, Y.; Savilov, S.V.; Lin, J. Pseudocapacitive Na-ion storage boosts high rate and areal capacity of self-branched 2D layered metal chalcogenide nanoarrays. ACS Nano 2016, 10, 10211–10219. [Google Scholar] [CrossRef]
- Yao, K.; Xu, Z.; Huang, J.; Ma, M.; Fu, L.; Shen, X.; Li, J.; Fu, M. Bundled defect-rich MoS2 for a high-rate and long-life sodium-ion battery: Achieving 3D diffusion of sodium ion by vacancies to improve kinetics. Small 2019, 15, 1805405. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, W.; Liu, T. A flexible free-standing defect-rich MoS2/graphene/carbon nanotube hybrid paper as a binder-free anode for high-performance lithium ion batteries. RSC Adv. 2015, 5, 43130–43140. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Cao, T.; He, F.; Shi, C.; He, C.; Ma, L.; Li, Q.; Li, J.; Zhao, N. Controllable graphene incorporation and defect engineering in MoS2-TiO2 based composites: Towards high-performance lithium-ion batteries anode materials. Nano Energy 2017, 33, 247–256. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, W.; Tjiu, W.W.; Liu, T. 3D porous hybrids of defect-rich MoS2/graphene nanosheets with excellent electrochemical performance as anode materials for lithium ion batteries. RSC Adv. 2015, 5, 34777–34787. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; He, F.; Shi, C.; He, C.; Li, J.; Zhao, N. 2D sandwich-like carbon-coated ultrathin TiO2@ defect-rich MoS2 hybrid nanosheets: Synergistic-effect-promoted electrochemical performance for lithium ion batteries. Nano Energy 2016, 26, 541–549. [Google Scholar] [CrossRef]
- Chen, B.; Lu, H.; Zhao, N.; Shi, C.; Liu, E.; He, C.; Ma, L. Facile synthesis and electrochemical properties of continuous porous spheres assembled from defect-rich, interlayer-expanded, and few-layered MoS2/C nanosheets for reversible lithium storage. J. Power Sources 2018, 387, 16–23. [Google Scholar] [CrossRef]
- Wang, J.; Luo, C.; Gao, T.; Langrock, A.; Mignerey, A.C.; Wang, C. An advanced MoS2/carbon anode for high-performance sodium-ion batteries. Small 2015, 11, 473–481. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Z.; Ma, Q.; Zhang, H. Novel structured transition metal dichalcogenide nanosheets. Chem. Soc. Rev. 2018, 47, 3301–3338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, D.; Wei, Q.; Ju, H.; Feng, J.; Zhu, J.; Mai, L.; Cairns, E.J.; Guo, J. Understanding the electrochemical reaction mechanism of VS2 nanosheets in lithium-ion cells by multiple in situ and ex situ x-ray spectroscopy. J. Phys. D Appl. Phys. 2018, 51, 494001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chowdari, B.; Wen, Z.; Jin, J.; Yang, J. Constructing highly oriented configuration by few-layer MoS2: Toward high-performance lithium-ion batteries and hydrogen evolution reactions. ACS Nano 2015, 9, 12464–12472. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, S.; Xiong, C.; Hu, G. In situ growth of 1T-MoS2 on liquid-exfoliated graphene: A unique graphene-like heterostructure for superior lithium storage. Carbon 2018, 133, 162–169. [Google Scholar] [CrossRef]
- Geng, X.; Jiao, Y.; Han, Y.; Mukhopadhyay, A.; Yang, L.; Zhu, H. Freestanding metallic 1T MoS2 with dual ion diffusion paths as high rate anode for sodium-ion batteries. Adv. Funct. Mater. 2017, 27, 1702998. [Google Scholar] [CrossRef]
- Lu, J.; Xia, G.; Gong, S.; Wang, C.; Jiang, P.; Lin, Z.; Wang, D.; Yang, Y.; Chen, Q. Metallic 1T phase MoS2 nanosheets decorated hollow cobalt sulfide polyhedra for high-performance lithium storage. J. Mater. Chem. A 2018, 6, 12613–12622. [Google Scholar] [CrossRef]
- Ding, W.; Hu, L.; Dai, J.; Tang, X.; Wei, R.; Sheng, Z.; Liang, C.; Shao, D.; Song, W.; Liu, Q. Highly ambient-stable 1T-MoS2 and 1T-WS2 by hydrothermal synthesis under high magnetic fields. ACS Nano 2019, 13, 1694–1702. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Gong, S.; Lv, F.; Wang, W.; Li, Y.; Luo, M.; Xing, Y.; Wang, Q.; Guo, S. Co-doped 1T-MoS2 nanosheets embedded in N, S-doped carbon nanobowls for high-rate and ultra-stable sodium-ion batteries. Nano Res. 2019, 12, 2218–2223. [Google Scholar] [CrossRef]
- Cai, L.; He, J.; Liu, Q.; Yao, T.; Chen, L.; Yan, W.; Hu, F.; Jiang, Y.; Zhao, Y.; Hu, T. Vacancy-induced ferromagnetism of MoS2 nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Z. Adsorption and diffusion of lithium on heteroatom-doped monolayer molybdenum disulfide. Appl. Surf. Sci. 2018, 455, 911–918. [Google Scholar] [CrossRef]
- Qin, S.; Lei, W.; Liu, D.; Chen, Y. Advanced N-doped mesoporous molybdenum disulfide nanosheets and the enhanced lithium-ion storage performance. J. Mater. Chem. A 2016, 4, 1440–1445. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, H.; Zhou, J.; Luo, Z.; Fang, G.; Liu, S.; Pan, A.; Liang, S. Nitrogen doped hollow MoS2/C nanospheres as anode for long-life sodium-ion batteries. Chem. Eng. J. 2017, 327, 522–529. [Google Scholar] [CrossRef]
- Wang, Y.; Von Lim, Y.; Huang, S.; Ding, M.; Kong, D.; Pei, Y.; Xu, T.; Shi, Y.; Li, X.; Yang, H.Y. Enhanced sodium storage kinetics by volume regulation and surface engineering via rationally designed hierarchical porous FeP@C/rGO. Nanoscale 2020, 12, 4341–4351. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, Q.; Chen, T.; Chen, W.; Ye, J.; Huang, J. Hydrothermal synthesis of Co-doped-MoS2/reduced graphene oxide hybrids with enhanced electrochemical lithium storage performances. Mater. Chem. Phys. 2018, 219, 399–410. [Google Scholar] [CrossRef]
- Chu, J.; Yu, Q.; Yang, D.; Xing, L.; Lao, C.-Y.; Wang, M.; Han, K.; Liu, Z.; Zhang, L.; Du, W. Thickness-control of ultrathin bimetallic Fe–Mo selenide@N-doped carbon core/shell “nano-crisps” for high-performance potassium-ion batteries. Appl. Mater. Today 2018, 13, 344–351. [Google Scholar] [CrossRef]
- Zheng, T.; Li, G.; Dong, J.; Sun, Q.; Meng, X. Self-assembled Mn-doped MoS2 hollow nanotubes with significantly enhanced sodium storage for high-performance sodium-ion batteries. Inorg. Chem. Front. 2018, 5, 1587–1593. [Google Scholar] [CrossRef]
- Sakthivel, M.; Sukanya, R.; Chen, S.-M.; Dinesh, B. Synthesis of two-dimensional Sr-Doped MoSe2 nanosheets and their application for efficient electrochemical reduction of metronidazole. J. Phys. Chem. C 2018, 122, 12474–12484. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, H.; Li, T.; Du, S.; Li, J.; Zhang, Y.; Yang, X. Vertically Oxygen-Incorporated MoS2 Nanosheets Coated on Carbon Fibers for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 35206–35215. [Google Scholar] [CrossRef]
- Cai, G.; Peng, L.; Ye, S.; Huang, Y.; Wang, G.; Zhang, X. Defect-rich MoS2(1−x)Se2x few-layer nanocomposites: A superior anode material for high-performance lithium-ion batteries. J. Mater. Chem. A 2019, 7, 9837–9843. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhi, G.; Gong, X.; Gao, Z.; Zhang, J. Relaxing volume stress and promoting active sites in vertically grown 2D layered mesoporous MoS2(1-x)Se2x/rGO composites with enhanced capability and stability for lithium ion batteries. Electrochim. Acta 2018, 268, 424–434. [Google Scholar] [CrossRef]
- Jia, G.; Chao, D.; Tiep, N.H.; Zhang, Z.; Fan, H.J. Intercalation Na-ion storage in two-dimensional MoS2−xSex and capacity enhancement by selenium substitution. Energy Storage Mater. 2018, 14, 136–142. [Google Scholar] [CrossRef]
- Ersan, F.; Gökoğlu, G.; Aktürk, E. Adsorption and Diffusion of Lithium on Monolayer Transition Metal Dichalcogenides (MoS2(1–x)Se2x) Alloys. J. Phys. Chem. C 2015, 119, 28648–28653. [Google Scholar] [CrossRef]
- Xiao, J.; Choi, D.; Cosimbescu, L.; Koech, P.; Liu, J.; Lemmon, J.P. Exfoliated MoS2 nanocomposite as an anode material for lithium ion batteries. Chem. Mater. 2010, 22, 4522–4524. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. In situ synthesis of MoS 2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries. Chem. Commun. 2011, 47, 4252–4254. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Wong, J.I.; Tan, A.Y.S.; Hsu, C.-L.; Li, L.-J.; Lu, Y.-C.; Yang, H.Y. Self-assembly of hierarchical MoSx/CNT nanocomposites (2< x< 3): Towards high performance anode materials for lithium ion batteries. Sci. Rep. 2013, 3, 2169. [Google Scholar]
- Yun, Q.; Li, L.; Hu, Z.; Lu, Q.; Chen, B.; Zhang, H. Layered transition metal dichalcogenide-based nanomaterials for electrochemical energy storage. Adv. Mater. 2020, 32, 1903826. [Google Scholar] [CrossRef]
- Sawicki, M.; Shaw, L.L. Advances and challenges of sodium ion batteries as post lithium ion batteries. RSC Adv. 2015, 5, 53129–53154. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Singh, G. MoS2/graphene composite paper for sodium-ion battery electrodes. ACS Nano 2014, 8, 1759–1770. [Google Scholar] [CrossRef] [Green Version]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, N.; Guziejewski, D.; Yuan, A.; Shah, S.A. Recent Advancement and Structural Engineering in Transition Metal Dichalcogenides for Alkali Metal Ions Batteries. Materials 2023, 16, 2559. https://doi.org/10.3390/ma16072559
Ullah N, Guziejewski D, Yuan A, Shah SA. Recent Advancement and Structural Engineering in Transition Metal Dichalcogenides for Alkali Metal Ions Batteries. Materials. 2023; 16(7):2559. https://doi.org/10.3390/ma16072559
Chicago/Turabian StyleUllah, Nabi, Dariusz Guziejewski, Aihua Yuan, and Sayyar Ali Shah. 2023. "Recent Advancement and Structural Engineering in Transition Metal Dichalcogenides for Alkali Metal Ions Batteries" Materials 16, no. 7: 2559. https://doi.org/10.3390/ma16072559
APA StyleUllah, N., Guziejewski, D., Yuan, A., & Shah, S. A. (2023). Recent Advancement and Structural Engineering in Transition Metal Dichalcogenides for Alkali Metal Ions Batteries. Materials, 16(7), 2559. https://doi.org/10.3390/ma16072559