Preparation of High-Stability Ceramic Slurry with Gel Behavior for Stereolithography 3D Printing
Abstract
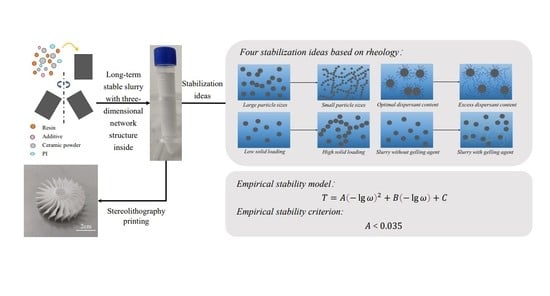
:1. Introduction
2. Experimental
2.1. Materials
2.2. Slurry Preparation
2.3. Three-Dimensional Printing and Post-Processing
2.4. Characterization
3. Results
3.1. Effects of Dispersant Species and Content on Slurry Viscosity
3.2. Effects of Particle Size on Loss Factor
3.3. Effects of Solid Loading on Loss Factor
3.4. Effects of Dispersant Content on Loss Factor
3.5. Effects of Dispersant Content on Curing Depth and Viscosity
3.6. Effects of Gelling Agent Content on Loss Factor
3.7. Post-Processing
3.8. Roughness and Defects
4. Discussion
4.1. Evolution of Structure inside the Slurry
4.1.1. Particle Size
4.1.2. Solid Loading
4.1.3. Dispersant Content
4.1.4. Gelling Agent Content
4.2. Empirical Stability Model
5. Conclusions
- (1)
- The particle size was negatively correlated with the stability of the internal three-dimensional network structure of the slurry. The 200 nm powder slurry could form a stable three-dimensional network structure at a 45 vol.% solid loading.
- (2)
- A U-shaped curve relating the stability of the three-dimensional network structure in the slurry and the solid loading was established. To create a three-dimensional network structure, the solid loading of a 200 nm slurry should be larger than 32.5 vol.% at least. Three-dimensional structure networks have the highest stability at 37.5 vol.%. Due to powder agglomeration, the stability of the slurry is compromised at larger solid contents.
- (3)
- At 1.5 times the ideal dispersant concentration, the slurry’s stability might last for 4 weeks. However, when the dispersant level increased, the strength of the three-dimensional network structure decreased, making the technique only effective for slurries with small particle sizes.
- (4)
- The addition of a gelling agent could significantly improve the stability and strength of the three-dimensional network structure in the slurry. For large-particle-size powder slurries, a gelling agent could be added to build a stable three-dimensional network structure to maintain slurry stability. However, the relationship between the slurry viscosity and gelling agent content must be balanced to ensure a smooth recoating process.
- (5)
- The experimental results provided the empirical stability model and the empirical stability factor A. When A is less than 0.035, the slurry is stable. The robust slurry stability test can be completed in less than 1 h.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive manufacturing of ceramics: Issues, potentialities, and opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Santoliquido, O.; Colombo, P.; Ortona, A. Additive manufacturing of ceramic components by digital light processing: A comparison between the “Bottom-up” and the “Top-down” approaches. J. Eur. Ceram. Soc. 2019, 39, 2140–2148. [Google Scholar] [CrossRef]
- Chaudhary, R.P.; Parameswaran, C.; Idrees, M.; Rasaki, A.S.; Liu, C.; Chen, Z.; Colombo, P. Additive manufacturing of polymer-derived ceramics: Materials, technologies, properties and potential applications. Prog. Mater. Sci. 2022, 128, 100969. [Google Scholar] [CrossRef]
- Lian, Q.; Yang, F.; Xin, H.; Li, D. Oxygen-controlled bottom-up mask-projection stereolithography for ceramic 3D printing. Ceram. Int. 2017, 43, 14956–14961. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, W.; Wu, H.; Song, X.; Chen, Y.; Cheng, L.; He, F.; Chen, S.; Wu, S. Preparation of a defect-free alumina cutting tool via additive manufacturing based on stereolithography—Optimization of the drying and debinding processes. Ceram. Int. 2016, 42, 11598–11602. [Google Scholar] [CrossRef]
- Bae, C.J.; Halloran, J.W. A segregation model study of suspension-based additive manufacturing. J. Eur. Ceram. Soc. 2018, 38, 5160–5166. [Google Scholar] [CrossRef]
- de Camargo, I.L.; Morais, M.M.; Fortulan, C.A.; Branciforti, M.C. A review on the rheological behavior and formulations of ceramic suspensions for vat photopolymerization. Ceram. Int. 2021, 47, 11906–11921. [Google Scholar] [CrossRef]
- Zhang, S.; Sha, N.; Zhao, Z. Surface modification of α-Al2O3 with dicarboxylic acids for the preparation of UV-curable ceramic suspensions. J. Eur. Ceram. Soc. 2017, 37, 1607–1616. [Google Scholar] [CrossRef]
- Abel, J.S.; Stangle, G.C.; Schilling, C.H.; Aksay, I.A. Sedimentation in flocculating colloidal suspensions. J. Mater. Res. 1994, 9, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Horn, R.G. Surface forces and their action in ceramic materials. J. Am. Ceram. Soc. 1990, 73, 1117–1135. [Google Scholar] [CrossRef]
- Zürcher, S.; Graule, T. Influence of dispersant structure on the rheological properties of highly-concentrated zirconia dispersions. J. Eur. Ceram. Soc. 2005, 25, 863–873. [Google Scholar] [CrossRef]
- Zhang, K.; He, R.; Xie, C.; Wang, G.; Ding, G.; Wang, M.; Song, W.; Fang, D. Photosensitive ZrO2 suspensions for stereolithography. Ceram. Int. 2019, 45, 12189–12195. [Google Scholar] [CrossRef]
- Cai, K.; Huang, Y.; Yang, J. Alumina gelcasting by using HEMA system. J. Eur. Ceram. Soc. 2005, 25, 1089–1093. [Google Scholar] [CrossRef]
- Wiesner, V.L.; Youngblood, J.P.; Trice, R.W. Room-temperature injection molding of aqueous alumina-polyvinylpyrrolidone suspensions. J. Eur. Ceram. Soc. 2014, 34, 453–463. [Google Scholar] [CrossRef]
- Borlaf, M.; Serra-Capdevila, A.; Colominas, C.; Graule, T. Development of UV-curable ZrO2 slurries for additive manufacturing (LCM-DLP) technology. J. Eur. Ceram. Soc. 2019, 39, 3797–3803. [Google Scholar] [CrossRef]
- Sun, J.; Binner, J.; Bai, J. Effect of surface treatment on the dispersion of nano zirconia particles in non-aqueous suspensions for stereolithography. J. Eur. Ceram. Soc. 2019, 39, 1660–1667. [Google Scholar] [CrossRef]
- Ding, G.; He, R.; Zhang, K.; Xia, M.; Feng, C.; Fang, D. Dispersion and stability of SiC ceramic slurry for stereolithography. Ceram. Int. 2020, 46, 4720–4729. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Z. The effect of the surfactants on the formulation of UV-curable SLA alumina suspension. Ceram. Int. 2017, 43, 4761–4767. [Google Scholar] [CrossRef]
- Macosko, C.W. Rheology: Principles, Measurements and Applications; Wiley-VCH: New York, NY, USA, 1994. [Google Scholar]
- Mezger, T. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers; Vincentz Network: Niedersachsen, Germany, 2022. [Google Scholar]
- Burgos-Montes, O.; Moreno, R. Stability of concentrated suspensions of Al2O3-SiO2 measured by multiple light scattering. J. Eur. Ceram. Soc. 2009, 29, 603–610. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Wen, L.; Cheng, L.; He, Y.; Xu, B.; Wu, Q.; Liu, S. Effects of particle size and color on photocuring performance of Si3N4 ceramic slurry by stereolithography. J. Eur. Ceram. Soc. 2021, 41, 2386–2394. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, C.; Wang, G.; He, R.; Ding, G.; Wang, M.; Dai, D.; Fang, D. High solid loading, low viscosity photosensitive Al2O3 slurry for stereolithography based additive manufacturing. Ceram. Int. 2019, 45, 203–208. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, W.; Wu, H.; Huang, R.; He, R.; Jiang, Q.; Chen, Y.; Ji, X.; Tian, Z.; Wu, S. Research into the mechanical properties, sintering mechanism and microstructure evolution of Al2O3-ZrO2 composites fabricated by a stereolithography-based 3D printing method. Mater. Chem. Phys. 2018, 207, 1–10. [Google Scholar] [CrossRef]
- Callister, W.D.J.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering: An Integrated Approach, 5th ed.; Wiley: New York, NY, USA, 2018. [Google Scholar]
- Griffith, M.L.; Halloran, J.W. Scattering of ultraviolet radiation in turbid suspensions. J. Appl. Phys. 1997, 81, 2538–2546. [Google Scholar] [CrossRef] [Green Version]
- Dou, R.; Tang, W.; Hu, K.; Wang, L. Ceramic paste for space stereolithography 3D printing technology in microgravity environment. J. Eur. Ceram. Soc. 2022, 42, 3968–3975. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, M.; Jiao, C.; Gu, J.; Xie, D.; Wang, C.; Tang, X.; Wei, Z.; Shen, L. Study on defect-free debinding green body of ceramic formed by DLP technology. Ceram. Int. 2020, 46, 2438–2446. [Google Scholar] [CrossRef]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics, 2nd ed.; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Wang, Z.; Huang, C.; Wang, J.; Zou, B. Development of a novel aqueous hydroxyapatite suspension for stereolithography applied to bone tissue engineering. Ceram. Int. 2019, 45, 3902–3909. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Liu, C.; Liu, Y.; Zhu, J.; Lao, C. Preparation of high solid loading and low viscosity ceramic slurries for photopolymerization-based 3D printing. Ceram. Int. 2019, 45, 11549–11557. [Google Scholar] [CrossRef]
- Pfaffinger, M.; Hartmann, M.; Schwentenwein, M.; Stampfl, J. Stabilization of tricalcium phosphate slurries against sedimentation for stereolithographic additive manufacturing and influence on the final mechanical properties. Int. J. Appl. Ceram. Technol. 2017, 14, 499–506. [Google Scholar] [CrossRef]












| d10 (μm) | d50 (μm) | d90 (μm) | |
|---|---|---|---|
| ZF2 | 0.14 | 0.21 | 0.50 |
| ZF5 | 0.26 | 0.55 | 0.92 |
| ZF10 | 0.59 | 1.03 | 1.83 |
| Materials | Contents |
|---|---|
| Zirconia powder | 30–45 vol.% of slurry |
| Dispersant | 1–7 wt% of Zirconia powder |
| Gelling agent | 0–2 wt% of slurry |
| Photosensitive resin | bal. |
| Solid Loading (vol.%) | Particle Sizes (μm) | Dispersant Content (wt%) | Gelling Agent Content (wt%) | |
|---|---|---|---|---|
| ZF2 | 45 | 200 | 6 | 0 |
| ZF5 | 45 | 500 | 5 | 0 |
| ZF10 | 45 | 1000 | 2 | 1.5 |
| Dispersant Content (wt%) | 4 | 5 | 6 | 7 |
|---|---|---|---|---|
| Agglomeration size (μm) | ~12 | ~7 | not evident | not evident |
| Dispersant Content (wt%) | Gelling Agent Content (wt%) | Empirical Stability Factor A | R2 | Stable Time (Day) | |
|---|---|---|---|---|---|
| ZF2 | 4 | 0 | 0.0930 | 0.9978 | ~14 |
| ZF2 | 5 | 0 | 0.0456 | 0.9975 | ~14 |
| ZF2 | 6 | 0 | 0.0310 | 0.9916 | >28 |
| ZF2 | 7 | 0 | 0.0335 | 0.9430 | >28 |
| ZF5 | 3 | 0 | 0.1892 | 0.9911 | <1 |
| ZF5 | 4 | 0 | 0.0936 | 0.9690 | <7 |
| ZF5 | 5 | 0 | 0.0253 | 0.9775 | >28 |
| ZF5 | 6 | 0 | 0.0227 | 0.9745 | >28 |
| ZF5 | 7 | 0 | 0.0256 | 0.9787 | >28 |
| ZF10 | 2 | 0 | 0.2200 | 0.9865 | <1 |
| ZF10 | 3 | 0 | 0.3248 | 0.9790 | <1 |
| ZF10 | 4 | 0 | 0.2591 | 0.9775 | <1 |
| ZF10 | 5 | 0 | 0.5350 | 0.9922 | <1 |
| ZF10 | 6 | 0 | 0.1786 | 0.9226 | <1 |
| ZF10 | 7 | 0 | 0.2897 | 0.8453 | <1 |
| ZF10 | 2 | 0.5 | 0.0387 | 0.9883 | ~7 |
| ZF10 | 2 | 1 | 0.0264 | 0.9781 | ~14 |
| ZF10 | 2 | 1.5 | −0.1436 | 0.6861 | >28 |
| ZF10 | 2 | 2 | −0.1212 | 0.8123 | >28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Chang, H.; Zhang, C.; Wu, Y.; Yang, R.; Zhang, X.; Zhai, Z. Preparation of High-Stability Ceramic Slurry with Gel Behavior for Stereolithography 3D Printing. Materials 2023, 16, 2816. https://doi.org/10.3390/ma16072816
Wang N, Chang H, Zhang C, Wu Y, Yang R, Zhang X, Zhai Z. Preparation of High-Stability Ceramic Slurry with Gel Behavior for Stereolithography 3D Printing. Materials. 2023; 16(7):2816. https://doi.org/10.3390/ma16072816
Chicago/Turabian StyleWang, Ning, Hai Chang, Chi Zhang, Yingna Wu, Rui Yang, Xing Zhang, and Zirong Zhai. 2023. "Preparation of High-Stability Ceramic Slurry with Gel Behavior for Stereolithography 3D Printing" Materials 16, no. 7: 2816. https://doi.org/10.3390/ma16072816
APA StyleWang, N., Chang, H., Zhang, C., Wu, Y., Yang, R., Zhang, X., & Zhai, Z. (2023). Preparation of High-Stability Ceramic Slurry with Gel Behavior for Stereolithography 3D Printing. Materials, 16(7), 2816. https://doi.org/10.3390/ma16072816