Formation of Self-Healing Organic Coatings for Corrosion Protection of Al Alloys by Dispersion of Spherical and Fibrous Capsules
Abstract
:1. Introduction




2. Experimental
2.1. Synthesis of Capsules Containing Healing Agent


2.2. Coating
2.3. Corrosion Test after Damaging
3. Results
3.1. Shape of Capsules Synthesized from Prepolymer Solution after Heating
3.2. Self-Healing Ability and Corrosion Protection of Coating Dispersed with Capsules with Spherical and Fibrous Shapes


4. Discussion
5. Conclusions
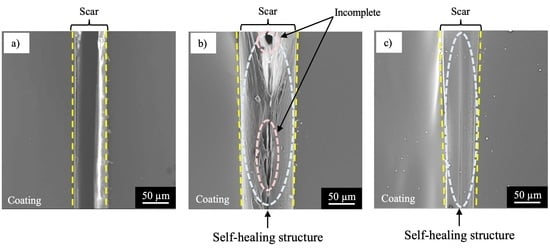
- The specimen with NC corroded heavily at the damaged area, suggesting no self-healing.
- The specimen with SHC-SC corroded partly at the damaged area, suggesting appreciable self-healing due to a small amount of healing agent flowing out from the broken capsules.
- The specimen with SHC-SFC did not corrode during the corrosion test after damage. This was due to repairing of the scar at the whole damaged area with healing agent, mainly from the fibrous capsules.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaw, B.A.; Kelly, R.G. What is Corrosion? Electrod1emical Soc. Interface 2006, 15, 24–26. [Google Scholar] [CrossRef]
- Roberge, P.R. Corrosion Engineering; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Kodama, T. Corrosion Cost Survey in Japan-Focusing on Transportation Industry. Corros. Sci. Technol. 2008, 7, 252–258. [Google Scholar]
- Ke, W.; Li, Z. Survey of Corrosion Cost in China and Preventive Strategies. Corros. Sci. Technol. 2008, 7, 259–264. [Google Scholar]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Bierwagen, G.P. Reflections on corrosion control by organic coatings. Prog. Org. Coat. 1996, 28, 43–48. [Google Scholar] [CrossRef]
- Feng, Y.; Teo, W.-K.; Siow, K.-S.; Gao, Z.; Tan, K.-L.; Hsieh, A.-K. Corrosion Protection of Copper by a Self- Assembled Monolayer of Alkanethiol. J. Electrochem. Soc. 1997, 144, 55–64. [Google Scholar] [CrossRef]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion protection by organic coatings: Electrochemical mechanism and novel methods of investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Muthkrishnan, S.; Venkatachari, G. Corrosion protection of steel by polyaniline blended coating. Electrochim. Acta 2006, 51, 6313–6319. [Google Scholar] [CrossRef]
- Gallegos-Melgar, A.; Serna, S.A.; Lázaro, I.; Gutiérrez-Castañeda, E.-J.; Mercado-Lemus, V.H.; Arcos-Gutierrez, H.; Hernández-Hernández, M.; Porcayo-Calderón, J.; Mayen, J.; Monroy, M.D.A. Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment. Coatings 2020, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Roberge, P.R. Handbook of Corrosion Engineering; Megraw-Hill: New York, NY, USA, 2019. [Google Scholar]
- Koch, G.H.; Brongers, M.P.H.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Costs and Preventive Strategies in the United States; FHWA-RD-01-156; National Technical Information Service: Springfield, VA, USA, 2001.
- Bhaskaran, R.; Palaniswamy, N.; Rengaswamy, N.S. A review of differing approaches used to estimate the cost of corrosion (and their relevance in the development of modern corrosion prevention andpcontrol strategies). Anti-Corros. Methods Mater. 2012, 52, 29–41. [Google Scholar] [CrossRef]
- Committee on Cost of Corrosion in Japan. Cost of Corrosion in Japan. Zair.-Kankyo 2020, 69, 283–306. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Stephenson, L.D.; Murray, J.N. Self-healing coatings for steel. Prog. Org. Coat. 2006, 55, 244–253. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Hatami Boura, S.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Szczygieł, I.; Szczygieł, B. Self-healing coatings in anti-corrosion applications. J. Mater. Sci. 2013, 48, 8041–8051. [Google Scholar] [CrossRef] [Green Version]
- Fayyad, E.M.; Almaadeed, M.A.; Jones, A.; Abdullah, A.M. Evaluation Techniques for the Corrosion Resistance of Self- Healing Coatings. Int. J. Electrochem. Sci. 2014, 9, 4989–5011. Available online: https://hdl.handle.net/10576/4594 (accessed on 14 March 2023).
- Son, H.M.; Kim, H.Y.; Park, S.M.; Lee, H.K. Ureolytic/Non-Ureolytic Bacteria Co-Cultured Self-Healing Agent for Cementitious Materials Crack Repair. Materials 2018, 11, 782. [Google Scholar] [CrossRef] [Green Version]
- Vila-Cortavitarte, M.; Jato-Espino, D.; Castro-Fresno, D.; Calzada-Pérez, M. Self-Healing Capacity of Asphalt Mixtures Including By-Products Both as Aggregates and Heating Inductors. Materials 2018, 11, 800. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Chen, H.; Zou, Y.; Ye, Y.; Zhao, H. Corrosion protective mechanism of smart graphene-based self-healing T coating on carbon steel. Corros. Sci. 2020, 174, 108825. [Google Scholar] [CrossRef]
- Yabuki, A.; Kanagaki, M.; Nishikawa, C.; Lee, J.H.; Fathona, I.W. Effective release of corrosion inhibitor by cellulose nanofibers and zeolite particles in self-healing coatings for corrosion protection. Prog. Org. Coat. 2021, 154, 106194. [Google Scholar] [CrossRef]
- Hirasawa, K.; Tomioka, Y.; Kawamura, M.; Hyono, A.; Chiba, M. Self-healing Coating by Using Pore of Porous Film Formed on Al Alloy Anodized and Effect of Pore-size on Self-healing Property of Coating. Zairyo-to-Kankyo 2022, 71, 63–69. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Fathona, I.W.; Yabuki, A. Self-healing polymer coating with efficient delivery for alginates and calcium nitrite to provide corrosion protection for carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130970. [Google Scholar] [CrossRef]
- Chiba, M.; Yamada, C.; Okuyama, H.; Sugiura, M.; Pletincx, S.; Verbruggen, H.; Hyono, A.; De Graeve, I.; Terryn, H.; Takahashi, H. Development of novel surface treatments for corrosion protection of aluminum: Self-repairing coatings. Corros. Rev. 2018, 36, 55–64. [Google Scholar] [CrossRef]
- Tsuji, Y.; Okuyama, H.; Yanagimoto, H.; Hyono, A.; Chiba, M.; Takahashi, H. Shape of Capsules Containing Healing Agent for Self-Healing Coating, by heat treatment. Zairyo-to-Kankyo 2019, 68, 152. [Google Scholar] [CrossRef]
- Qin, G.; Na, J.; Mu, W.; Tan, W. Effect of thermal cycling on the degradation of adhesively bonded CFRP/ aluminum alloy joints for automobiles. Int. J. Adhes. Adhes. 2019, 95, 102439. [Google Scholar] [CrossRef]
- Singh, H.; Brar, G.S.; Kumar, H.; Aggarwal, V. A review on metal matrix composite for automobile applications. Mater. Today: Proc. 2021, 43, 320–325. [Google Scholar] [CrossRef]
- Sondari, D.; Septevani, A.A.; Randy, A.; Triwulandari, E. Polyurethane microcapsule with glycerol as the polyol component for encapsulated self healing agent. Int. J. Eng. Technol. 2010, 2, 466–471. [Google Scholar]
- Yang, J.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of Isocyanates for Self-Healing Polymers. Macromolecules 2008, 41, 9650–9655. [Google Scholar] [CrossRef]
- Chiba, M.; Nakayama, Y.; Hiraga, T.; Takahashi, H.; Shibata, Y. Synergistic effects of Cl− and Cu2+ ions on corrosion of pure Al and Al alloys in aqueous solutions at 363 K. Surf. Interface Anal. 2013, 45, 1626–1630. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiba, M.; Tsuji, Y.; Takada, R.; Eguchi, Y.; Takahashi, H. Formation of Self-Healing Organic Coatings for Corrosion Protection of Al Alloys by Dispersion of Spherical and Fibrous Capsules. Materials 2023, 16, 3018. https://doi.org/10.3390/ma16083018
Chiba M, Tsuji Y, Takada R, Eguchi Y, Takahashi H. Formation of Self-Healing Organic Coatings for Corrosion Protection of Al Alloys by Dispersion of Spherical and Fibrous Capsules. Materials. 2023; 16(8):3018. https://doi.org/10.3390/ma16083018
Chicago/Turabian StyleChiba, Makoto, Yuki Tsuji, Rin Takada, Yuri Eguchi, and Hideaki Takahashi. 2023. "Formation of Self-Healing Organic Coatings for Corrosion Protection of Al Alloys by Dispersion of Spherical and Fibrous Capsules" Materials 16, no. 8: 3018. https://doi.org/10.3390/ma16083018
APA StyleChiba, M., Tsuji, Y., Takada, R., Eguchi, Y., & Takahashi, H. (2023). Formation of Self-Healing Organic Coatings for Corrosion Protection of Al Alloys by Dispersion of Spherical and Fibrous Capsules. Materials, 16(8), 3018. https://doi.org/10.3390/ma16083018