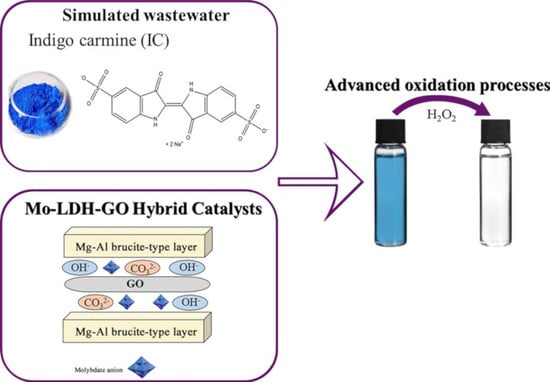
Mo-LDH-GO Hybrid Catalysts for Indigo Carmine Advanced Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Catalytic Tests
3. Results
3.1. Characterization of the Materials
3.1.1. Chemical Composition and Acid-Base Properties
3.1.2. Powder XRD Characterization
3.1.3. Characterization by Infrared Spectroscopy
3.1.4. Characterization by Raman Spectroscopy
3.1.5. Characterization Using SEM Microscopy
3.1.6. Textural Characterization
3.2. Catalytic Tests Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joel, E.F.; Lujaniené, G. Progress in Graphene Oxide Hybrids for Environmental Applications. Environments 2022, 9, 153. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Marcu, I.-C.; Tichit, D. Chapter 4. Recent Innovative Developments of Layered Double Hydroxide-Based Hybrids and Nanocomposite Catalysts. In Series on Chemistry, Energy and the Environment: Progress in Layered Double Hydroxides from Synthesis to New Applications; Nocchetti, M., Costantino, U., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2022; Volume 8, pp. 189–362. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Gunjakar, J.L.; Kim, I.Y.; Lee, J.M.; Lee, N.-S.; Hwang, S.-J. Self-assembly of layered double hydroxide 2D nanoplates with graphene nanosheets: An effective way to improve the photocatalytic activity of 2D nanostructured materials for visible light-induced O2 generation. Energy Environ. Sci. 2013, 6, 1008–1017. [Google Scholar] [CrossRef]
- Motlagh, P.Y.; Khataee, A.; Sadeghi Rad, T.; Hassani, A.; Joo, S.W. Fabrication of ZnFe-layered double hydroxides with graphene oxide for efficient visible light photocatalytic performance. J. Taiwan Inst. Chem. Eng. 2019, 101, 186–203. [Google Scholar] [CrossRef]
- Motlagh, P.Y.; Khataee, A.; Hassani, A.; Sadeghi Rad, T. ZnFe-LDH/GO nanocomposite coated on the glass support as a highly efficient catalyst for visible light photodegradation of an emerging pollutant. J. Mol. Liq. 2020, 302, 112532. [Google Scholar] [CrossRef]
- Sadeghi, R.T.; Yazici, E.S.; Khataee, A.; Gengec, E.; Kobya, M. Nanoarchitecture of graphene nanosheets decorated with NiCr layered double hydroxide for sonophotocatalytic degradation of refractory antibiotics. Environ. Res. 2022, 214, 113788. [Google Scholar] [CrossRef]
- Asif, M.; Saeed, M.; Zafar, M.; Amjad, U.-E.-S.; Razzaq, A.; Young Kim, W. Development of Co-Al LDH/GO composite photocatalyst for enhanced degradation of textile pollutant under visible light irradiation. Results Phys. 2022, 42, 105997. [Google Scholar] [CrossRef]
- Wang, K.; Miao, C.; Liu, Y.; Cai, L.; Jones, W.; Fan, J.; Li, D.; Feng, J. Vacancy enriched ultrathin TiMgAl-layered double hydroxide/graphene oxides composites as highly efficient visible-light catalysts for CO2 reduction. Appl. Catal. B. 2020, 270, 118878. [Google Scholar] [CrossRef]
- Rezaei, B.; Khosropour, H.; Ensafi, A.A.; Dinari, M.; Nabiyan, A. A new electrochemical sensor for the simultaneous determination of guanine and adenine: Using a NiAl-layered double hydroxide/graphene oxide-multi wall carbon nanotube modified glassy carbon electrode. RSC Adv. 2015, 5, 75756–75765. [Google Scholar] [CrossRef]
- Rezaei, B.; Heidarbeigy, M.; Ensafi, A.A.; Dinari, M. Electrochemical Determination of Papaverine on Mg-Al Layered Double Hydroxide/ Graphene Oxide and CNT Modified Carbon Paste Electrode. IEEE Sens. J. 2016, 16, 3496–3503. [Google Scholar] [CrossRef]
- Zhou, J.; Min, M.; Liu, Y.; Tang, J.; Tang, W. Layered assembly of NiMn-layered double hydroxide on graphene oxide for enhanced non-enzymatic sugars and hydrogen peroxide detection. Sens. Actuators B Chem. 2018, 260, 408–417. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Nyamori, V.O. Layered double hydroxide- and graphene-based hierarchical nanocomposites: Synthetic strategies and promising applications in energy conversion and conservation. Nano Res. 2016, 9, 3598–3621. [Google Scholar] [CrossRef]
- Cao, L.; Ma, Y.; Song, A.; Bai, L.; Zhang, P.; Li, X.; Shao, G. Stable composite of flower-like NiFe-layered double hydroxide nucleated on graphene oxide as an effective catalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 5912–5920. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, P.; Xie, R.; Chen, L.; Li, M.; Li, J.; Ji, B.; Hu, Z.; Li, J.; Song, L.; et al. Controlled Self-Assembled NiFe Layered Double Hydroxides/Reduced Graphene Oxide Nanohybrids Based on the Solid-Phase Exfoliation Strategy as an Excellent Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 13545–13556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, T.; Wu, N.; Zhou, B.; Yan, Y.; Ye, Y.; Gong, J.; Yang, S. Bimetallic electron-induced phase transformation of CoNi LDH-GO for high oxygen evolution and supercapacitor performance. Sci. China Mater. 2023, 66, 577–586. [Google Scholar] [CrossRef]
- Altaf, N.; Liang, S.; Iqbal, R.; Hayat, M.; Reina, T.R.; Wang, Q. Cu-CuOx/rGO catalyst derived from hybrid LDH/GO with enhanced C2H4 selectivity by CO2 electrochemical reduction. J. CO2 Util. 2020, 40, 101205. [Google Scholar] [CrossRef]
- Yang, H.; Guo, T.; Yin, D.; Liu, Q.; Zhang, X. A high-efficiency noble metal-free electrocatalyst of cobalt-iron layer double hydroxides nanorods coupled with graphene oxides grown on a nickel foam towards methanol electrooxidation. J. Taiwan Inst. Chem. Eng. 2020, 112, 212–221. [Google Scholar] [CrossRef]
- Mei, X.; Wang, J.; Yang, R.; Yan, Q.; Wang, Q. Synthesis of Pt doped Mg-Al layered double oxide/graphene oxide hybrid as novel NOx storage-reduction catalyst. RSC Adv. 2015, 5, 78061–78070. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Menzel, R.; Wang, Y.; Garcia-Gallastegui, A.; Bawaked, S.M.; Obaid, A.Y.; Basahel, S.N.; Mokhtar, M. Graphene-oxide-supported CuAl and CoAl layered double hydroxides as enhanced catalysts for carbon-carbon coupling via Ullmann reaction. J. Solid State Chem. 2017, 246, 130–137. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Zhao, Y.; Miras, H.N.; Song, Y.-F. Precise Control of the Oriented Layered Double Hydroxide Nanosheets Growth on Graphene Oxides Leading to Efficient Catalysts for Cascade Reactions. ChemCatChem 2019, 11, 5466–5474. [Google Scholar] [CrossRef]
- Stamate, A.-E.; Pavel, O.D.; Zăvoianu, R.; Brezeştean, I.; Ciorîță, A.; Bîrjega, R.; Neubauer, K.; Koeckritz, A.; Marcu, I.-C. Ce-containing MgAl-layered double hydroxide-graphene oxide hybrid materials as multifunctional catalysts for organic transformations. Materials 2021, 14, 7457. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xiao, L.; Yuan, S.; Wang, W.; Zhan, X.; Hu, Z.-H. Activation of peroxymonosulfate by CoFeNi layered double hydroxide/graphene oxide (LDH/GO) for the degradation of gatifloxacin. Sep. Purif. Technol. 2021, 255, 117685. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B. Self-assembly of graphene oxide aerogels by layered double hydroxides cross-linking and their application in water purification. J. Mater. Chem. 2014, 2, 8941–8951. [Google Scholar] [CrossRef]
- Sharifi-Bonab, M.; Aber, S.; Salari, D.; Khodam, F. Synthesis of CoZnAl-layered double hydroxide/graphene oxide nanocomposite for the removal of methylene blue: Kinetic, thermodynamic, and isotherm studies Environmental. Environ. Prog. Sustain. 2020, 39, 13316. [Google Scholar] [CrossRef]
- Rashed, S.H.; Abd-Elhamid, A.I.; Abdalkarim, S.Y.H.; El-Sayed, R.H.; El-Bardan, A.A.; Soliman, H.M.A.; Nayl, A.A. Preparation and characterization of layered-double hydroxides decorated on graphene oxide for dye removal from aqueous solution. J. Mater. Res. Technol. 2022, 17, 2782–2795. [Google Scholar] [CrossRef]
- Lu, P.; Liang, S.; Zhou, T.; Mei, X.; Zhang, Y.; Zhang, C.; Umar, A.; Wang, Q. Layered double hydroxide/graphene oxide hybrid incorporated polysulfone substrate for thin-film nanocomposite forward osmosis membranes. RSC Adv. 2016, 6, 56599–56609. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Z.; Peng, Y.; Zhu, L. Environmentally friendly electrostatically driven self-assembled LDH/GO/PVDF composite membrane for water treatment. Appl. Clay Sci. 2019, 183, 105322. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, A.S.; Tran, B.A.; Vu, K.O.; Tran, D.L.; Phan, T.T.; Scharnagl, N.; Zheludkevich, M.L.; To, T.X.H. Molybdate intercalated hydrotalcite/graphene oxide composite as corrosion inhibitor for carbon steel. Surf. Coat. Technol. 2020, 399, 126165. [Google Scholar] [CrossRef]
- van Laar, F.M.P.R.; De Vos, D.E.; Pierard, F.; Kirsch-De Mesmaeker, A.; Fiermans, L.; Jacobs, P.A. Generation of Singlet Molecular Oxygen from H2O2 with Molybdate-Exchanged Layered Double Hydroxides: Effects of Catalyst Composition and Reaction Conditions. J. Catal. 2001, 197, 139–150. [Google Scholar] [CrossRef]
- Zăvoianu, R.; Bîrjega, R.; Pavel, O.D.; Cruceanu, A.; Alifanti, M. Hydrotalcite like compounds with low Mo-loading active catalysts for selective oxidation of cyclohexene with hydrogen peroxide. Appl. Catal. A Gen. 2005, 286, 211–220. [Google Scholar] [CrossRef]
- Zăvoianu, R.; Cruceanu, A.; Pavel, O.D.; Angelescu, E.; Soares Dias, A.P.; Birjega, R. Oxidation of tert-butanethiol with air using Mo containing hydrotalcite-like compounds and their derived mixed oxides as catalysts. React. Kinet. Mech. Catal. 2012, 105, 145–162. [Google Scholar] [CrossRef]
- Klemkaitè-Ramanauskè, K.; Žilinskas, A.; Taraškevičius, R.; Khinsky, A.; Kareiva, A. Preparation of Mg/Al layered double hydroxide (LDH) with structurally embedded molybdate ions and application as a catalyst for the synthesis of 2-adamantylidene(phenyl)amine Schiff base. Polyhedron 2014, 68, 340–345. [Google Scholar] [CrossRef]
- van Laar, F.M.P.R.; De Vos, D.E.; Vanoppen, D.; Sels, B.; Jacobs, P.A. Heterogeneous molybdate catalysts for the generation of singlet molecular oxygen (1Δg) from H2O2. Chem. Commun. 1998, 2, 267–268. [Google Scholar] [CrossRef]
- Maciuca, A.-L.; Ciocan, C.-E.; Dumitriu, E.; Fajula, F.; Hulea, V. V-, Mo- and W-containing layered double hydroxides as effective catalysts for mild oxidation of thioethers and thiophenes with H2O2, V-, Mo- and W-containing layered double hydroxides as effective catalysts for mild oxidation of thioethers and thiophenes with H2O2. Catal. Today 2008, 138, 33–37. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. Sci. Eng. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- El Gaini, L.; Lakraimi, M.; Sebbar, E.; Meghea, A.; Bakasse, M. Removal of indigo carmine dye from water to Mg–Al–CO3-calcined layered double hydroxides. J. Hazard. Mater. 2009, 161, 627–632. [Google Scholar] [CrossRef]
- Drumond Chequer, F.M.; de Oliveira, G.A.R.; Anastacio Ferraz, E.R.; Carvalho, J.; Boldrin Zanoni, M.V.; de Oliveir, D.P. Textile Dyes: Dyeing Process and Environmental Impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Senthil Kumar, P.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Asaithambi, P.; Yesuf, M.B.; Govindarajan, R.; Hariharan, N.M.; Thangavelu, P.; Alemayehu, E. A review of hybrid processes development based on electrochemical and advanced oxidation processes for the treatment of industrial wastewater. Int. J. Chem. Eng. 2022, 2022, 1105376. [Google Scholar] [CrossRef]
- Asaithambi, P.; Saravanathamizhan, R.; Matheswaran, M. Comparison of treatment and energy efficiency of advanced oxidation processes for the distillery wastewater. Int. J. Environ. Sci. Technol. 2015, 12, 2213–2220. [Google Scholar] [CrossRef]
- Sewnet, A.; Abebe, M.; Asaithambi, P.; Alemayehu, E. Visible-lightdriven g-C3N4/TiO2 based heterojunction nanocomposites for photocatalytic degradation of organic dyes in wastewater: A review. Air Soil Water Res. 2022, 15, 1–23. [Google Scholar] [CrossRef]
- Ribeiro, A.R.L.; Hermosilla, D.; Mueses, M.A.; Xiao, R.; Mantzavinos, D. Advanced oxidation technologies for water/wastewater treatment: Advances, gaps and challenges—Editorial. Adv. Chem. Eng. 2022, 10, 100272. [Google Scholar] [CrossRef]
- Wambuguh, D.; Chianellia, R.R. Indigo dye waste recovery from blue denim textile effluent: A by-product synergy approach. New J. Chem. 2008, 32, 2189–2194. [Google Scholar] [CrossRef]
- Jabs, C.F.I.; Drutz, H.P. The role of intra-operative cystoscopy in prolapsed and incontinence surgery. Am. J. Obstet. Gynecol. 2001, 185, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Sannohe, Y.; Araki, S.; Inutsuka, S. Intra-arterial dye method with vasomotors (PIAD method) applied for the endoscopic diagnosis of gastric cancer and the side effects of indigo carmine. Endoscopy 1982, 14, 119–123. [Google Scholar] [CrossRef]
- Konig, J. Food colour additives of synthetic origin. In Colour Additives for Foods and Beverages; Scotter, M.J., Ed.; Woodhead Publ.: Cambridge UK, 2015; Chapter 2; pp. 35–60. [Google Scholar] [CrossRef]
- Vautier, M.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation of Dyes in Water: Case Study of Indigo and of Indigo Carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- Barka, N.; Assabbane, A.; Nounah, A.; Aît Ichou, Y. Photocatalytic degradation of indigo carmine in aqueous solution by TiO2-coated non-woven fibres. J. Hazard. Mater. 2008, 152, 1054–1059. [Google Scholar] [CrossRef]
- Palma-Goyes, R.E.; Silva-Agredo, J.; González, I.; Torres-Palma, R.A. Comparative degradation of indigo carmine by electrochemical oxidation and advanced oxidation processes. Electrochim. Acta 2014, 140, 427–433. [Google Scholar] [CrossRef]
- Vidya Lekshmi, K.P.; Yesodharan, S.; Yesodharan, E.P. MnO2 efficiently removes indigo carmine dyes from polluted water. Heliyon 2018, 4, e00897. [Google Scholar] [CrossRef] [PubMed]
- Tabti, S.; Benchettara, A.; Smaili, F.; Benchettara, A.; Berrabah, S.E. Electrodeposition of lead dioxide on Fe electrode: Application to the degradation of Indigo Carmine dye. J. Appl. Electrochem. 2022, 52, 1207–1217. [Google Scholar] [CrossRef]
- Kuncser, A.C.; Rostas, A.M.; Zavoianu, R.; Pavel, O.D.; Vlaicu, I.D.; Badea, M.; Culita, D.C.; Tirsoaga, A.; Olar, R. Synthesis and Characterization of Hematite-Based Nanocomposites as Promising Catalysts for Indigo Carmine Oxidation. Nanomaterials 2022, 12, 2511. [Google Scholar] [CrossRef] [PubMed]
- Zăvoianu, R.; Pavel, O.D.; Cruceanu, A.; Florea, M.; Bîrjega, R. Functional layered double hydroxides and their catalytic activity for 1,4-addition of n-octanol to 2-propenonitrile. Appl. Clay Sci. 2017, 146, 411–422. [Google Scholar] [CrossRef]
- Álvarez, M.; Crivoi, D.; Medina, F.; Tichit, D. Synthesis of Chalcone Using LDH/Graphene Nanocatalysts of Different Compositions. Chemengineering 2019, 3, 29. [Google Scholar] [CrossRef]
- Frost, R.L.; Scholz, R.; López, A.; Theiss, F.L. Vibrational spectroscopic study of the natural layered double hydroxide manasseite now defined as hydrotalcite-2H—Mg6Al2(OH)16[CO3]·4H2O. Spectrochim. Acta A 2014, 118, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, J.; Zhang, M.; Yang, P.; Yang, L.; Cao, L.; Li, J. One-step synthesis of lamellar molybdate pillared hydrotalcite and its application for AZ31 Mg alloy protection. Solid State Sci. 2009, 11, 376–381. [Google Scholar] [CrossRef]
- Davantès, A.; Lefèvre, G.J. In Situ Real Time Infrared Spectroscopy of Sorption of (Poly)molybdate Ions into Layered Double Hydroxides. Phys. Chem. A 2013, 117, 12922–12929. [Google Scholar] [CrossRef]
- Khalili, D. Graphene oxide: A promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. New J. Chem. 2016, 40, 2547–2553. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- NTPA-001/2002 norms (updated in 2007) regarding the admissible concentration of pollutants in industrial and domestic waste waters for their discharge in natural receptors. MOF 2002, 187, 1–45, MOF 2007, 210, 1–10.









| Catalysts | Metal Content (wt.%) | Atomic Ratios | |||
|---|---|---|---|---|---|
| Mg2+ | Al3+ | Mo | Mg/Al | Mo/Al | |
| HTMo | 15.9 | 6.1 | 10.5 | 2.90 | 0.48 |
| HTMo-5GO | 14.5 | 5.7 | 8.7 | 2.83 | 0.43 |
| HTMo-10GO | 13.6 | 5.4 | 7.6 | 2.80 | 0.40 |
| HTMo-15GO | 12.6 | 5.0 | 6.9 | 2.80 | 0.39 |
| HTMo-20GO | 11.3 | 4.6 | 4.2 | 2.73 | 0.26 |
| HTMo-25GO | 10.0 | 4.1 | 3.6 | 2.71 | 0.25 |
| Catalysts | Total Acid Sites (mmol Py/g) | HB 1 | Total Base Sites (mmol AA/g) | SB 2 | Base/Acid Sites Ratio |
|---|---|---|---|---|---|
| (%) | (%) | ||||
| HTMo | 0.15 | 10.1 | 0.20 | 25.1 | 1.33 |
| HTMo-5GO | 0.18 | 14.2 | 0.28 | 20.2 | 1.55 |
| HTMo-10GO | 0.25 | 18.4 | 0.40 | 17.4 | 1.60 |
| HTMo-15GO | 0.34 | 24.6 | 0.48 | 14.7 | 1.65 |
| HTMo-20GO | 0.20 | 21.3 | 0.52 | 10.8 | 2.40 |
| HTMo-25GO | 0.30 | 27.5 | 0.56 | 8.2 | 1.73 |
| GO [24] | 0.77 | 31.2 | 0.06 | 0 | 0.08 |
| Samples | Lattice Parameters | Crystallite Sizes | I003/I110 | I110(HTMO)/ I110(HTMo-xGO) | ||
|---|---|---|---|---|---|---|
| a (Å) | c (Å) | D003 (nm) | D110 (nm) | |||
| HTMo | 3.061 | 23.814 | 12.7 | 6.8 | 4.4 | 1.00 |
| HTMo-5GO | 3.058 | 23.582 | 10.4 | 6.5 | 4.2 | 0.85 |
| HTMo-10GO | 3.059 | 23.509 | 12.4 | 8.3 | 5.2 | 0.84 |
| HTMo-15GO | 3.058 | 23.326 | 12.3 | 8.3 | 4.7 | 0.99 |
| HTMo-20GO | 3.055 | 23.232 | 13.7 | 7.2 | 6.4 | 0.94 |
| HTMo-25GO | 3.054 | 23.271 | 15.0 | 6.9 | 6.7 | 0.92 |
| Samples | Ssp BET (m2/g) | t-Plot Micropore Area (m2/g) | t-Plot External Surface Area (m2/g) | BJH Adsorption Average Pore Width (nm) | BJH Adsorption Cumulative Volume of Pores (cm3/g) | Pore Size 1 nm |
|---|---|---|---|---|---|---|
| HTMo | 4.1 | 0.3 | 3.8 | 17.5 | 0.020 | 2.7 and 18.1 |
| HTMo-5GO | 22.7 | 2.0 | 20.8 | 8.6 | 0.057 | 2.7 and 9.1 |
| HTMo-10GO | 46.5 | 3.5 | 43.0 | 8.5 | 0.116 | 2.4 and 9.0 |
| HTMo-15GO | 82.9 | 5.0 | 77.9 | 8.6 | 0.209 | 2.7 and 9.0 |
| HTMo-20GO | 61.6 | 1.6 | 60.0 | 10.2 | 0.185 | 2.4 and 10.9 |
| HTMo-25GO | 80.9 | 2.5 | 78.4 | 8.0 | 1.197 | 2.6 and 9.0 |
| GO [24] | 79.8 | 24.5 | 55.3 | 5.5 | 0.059 | 3.9 |
| Catalysts | Molar Ratios H2O2/IC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 32.1 | 40 | 48 | 56 | 64 | ||||||
| IC Conv. (%) | H2O2 Conv. (%) | IC Conv. (%) | H2O2 Conv. (%) | IC Conv. (%) | H2O2 Conv. (%) | IC Conv. (%) | H2O2 Conv. (%) | IC Conv. (%) | H2O2 Conv. (%) | |
| HTMo | 66.1 | 70.1 | 71.3 | 61.2 | 74.5 | 53.8 | 79.1 | 49.3 | 82.8 | 45.5 |
| HTMo-5GO | 82.7 | 86.7 | 83.7 | 71.2 | 87.6 | 62.6 | 89.6 | 55.4 | 90.4 | 49.3 |
| HTMo-10GO | 83.4 | 87.4 | 87.6 | 74.1 | 89.8 | 64.1 | 91.4 | 56.4 | 91.8 | 50.0 |
| HTMo-15GO | 86.1 | 98.6 | 90.3 | 80.1 | 90.7 | 68.6 | 91.8 | 58.9 | 92.3 | 51.1 |
| HTMo-20GO | 94.6 | 95.4 | 94.8 | 78.1 | 96.6 | 66.5 | 95.7 | 57.5 | 95.9 | 52.2 |
| HTMo-25GO | 91.4 | 90.1 | 92.3 | 76.5 | 93.5 | 64.7 | 93.4 | 56.6 | 93.7 | 53.0 |
| GO | 12.3 | 16.3 | 16.2 | 17.0 | 20.2 | 17.5 | 22.1 | 16.7 | 23.5 | 15.8 |
| blank | 1.5 | 5.5 | 3.1 | 6.5 | 4.2 | 6.8 | 5.3 | 7.0 | 6.4 | 7.2 |
| Catalysts | ICo = 15 × 10−3 M | ICo = 90 × 10−3 M | ||
|---|---|---|---|---|
| IC Conv. (%) | H2O2 Conv. (%) | IC Conv. (%) | H2O2 Conv. (%) | |
| HTMo | 78.4 | 56.4 | 68.7 | 55.7 |
| HTMo-5GO | 90.1 | 64.3 | 81.2 | 64.9 |
| HTMo-10GO | 91.4 | 65.1 | 84.7 | 67.5 |
| HTMo-15GO | 92.2 | 65.7 | 85.4 | 68.1 |
| HTMo-20GO | 98.2 | 69.7 | 95.4 | 75.6 |
| HTMo-25GO | 96.4 | 68.5 | 88.3 | 70.2 |
| GO | 35.4 | 27.7 | 26.5 | 24.1 |
| blank | 4.6 | 7.1 | 3.5 | 6.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, O.D.; Stamate, A.-E.; Zăvoianu, R.; Cruceanu, A.; Tirsoaga, A.; Bîrjega, R.; Brezeștean, I.A.; Ciorîță, A.; Culiță, D.C.; Dias, A.P.S. Mo-LDH-GO Hybrid Catalysts for Indigo Carmine Advanced Oxidation. Materials 2023, 16, 3025. https://doi.org/10.3390/ma16083025
Pavel OD, Stamate A-E, Zăvoianu R, Cruceanu A, Tirsoaga A, Bîrjega R, Brezeștean IA, Ciorîță A, Culiță DC, Dias APS. Mo-LDH-GO Hybrid Catalysts for Indigo Carmine Advanced Oxidation. Materials. 2023; 16(8):3025. https://doi.org/10.3390/ma16083025
Chicago/Turabian StylePavel, Octavian Dumitru, Alexandra-Elisabeta Stamate, Rodica Zăvoianu, Anca Cruceanu, Alina Tirsoaga, Ruxandra Bîrjega, Ioana Andreea Brezeștean, Alexandra Ciorîță, Daniela Cristina Culiță, and Ana Paula Soares Dias. 2023. "Mo-LDH-GO Hybrid Catalysts for Indigo Carmine Advanced Oxidation" Materials 16, no. 8: 3025. https://doi.org/10.3390/ma16083025
APA StylePavel, O. D., Stamate, A. -E., Zăvoianu, R., Cruceanu, A., Tirsoaga, A., Bîrjega, R., Brezeștean, I. A., Ciorîță, A., Culiță, D. C., & Dias, A. P. S. (2023). Mo-LDH-GO Hybrid Catalysts for Indigo Carmine Advanced Oxidation. Materials, 16(8), 3025. https://doi.org/10.3390/ma16083025