Miconazole Nitrate Microparticles in Lidocaine Loaded Films as a Treatment for Oropharyngeal Candidiasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
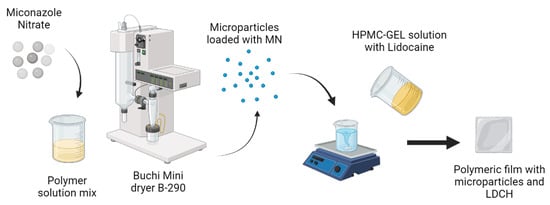
2.2. Preparation of Microparticles
2.3. Lidocaine Films Loaded Microparticles
2.4. High-Performance Liquid Chromatography (HPLC)
2.5. Microparticles Loaded MN—Encapsulation Efficiency
2.6. Films Loaded Microparticles: Thickness and Folding Endurance
2.7. Mechanical Properties
2.8. In Vitro Mucoadhesive Strength
2.9. Swelling Index
2.10. Morphology Analysis and Size Determination by Scanning Electron Microscopy
2.11. Thermal Analysis
2.12. X-ray Diffraction
2.13. Dissolution Studies
2.14. Halo Zone Test over Time
2.15. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation Efficiency of Microparticles
3.2. Film Thickness, Folding Endurance and Mechanical Properties
3.3. Swelling Index
3.4. In Vitro Mucoadhesive Strength
3.5. Morphology Analysis and Size Determination by Scanning Electron Microscopy
3.6. Thermal Characterization
3.7. X-ray Diffraction
3.8. Dissolution Studies
3.9. Halo Zone Test over Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spampinato, C.; Leonardi, D. Candida Infections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. BioMed Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef]
- Garcia-Cuesta, C.; Sarrion-Pérez, M.G.; Bagán, J.V. Current Treatment of Oral Candidiasis: A Literature Review. J. Clin. Exp. Dent. 2014, 6, e576. [Google Scholar] [CrossRef]
- Oji, C.; Chukwuneke, F. Evaluation and Treatment of Oral Candidiasis in HIV/AIDS Patients in Enugu, Nigeria. Oral Maxillofac. Surg. 2008, 12, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Yadav, N.P.; Sinha, P.; Mishra, N.; Luqman, S.; Dwivedi, H.; Kymonil, K.M.; Saraf, S.A. Development of Cellulosic Polymer Based Gel of Novel Ternary Mixture of Miconazole Nitrate for Buccal Delivery. Carbohydr. Polym. 2014, 103, 126–133. [Google Scholar] [CrossRef]
- De Caro, V.; Murgia, D.; Seidita, F.; Bologna, E.; Alotta, G.; Zingales, M.; Campisi, G. Enhanced In Situ Availability of Aphanizomenon Flos-Aquae Constituents Entrapped in Buccal Films for the Treatment of Oxidative Stress-Related Oral Diseases: Biomechanical Characterization and In Vitro/Ex Vivo Evaluation. Pharmaceutics 2019, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Mady, O.Y.; Donia, A.M.; Al-Madboly, L.A. Miconazole-Urea in a Buccal Film as a New Trend for Treatment of Resistant Mouth Fungal White Patches. Front. Microbiol. 2018, 9, 837. [Google Scholar] [CrossRef]
- De Caro, V.; Giannola, L.I.; Di Prima, G. Solid and Semisolid Innovative Formulations Containing Miconazole-Loaded Solid Lipid Microparticles to Promote Drug Entrapment into the Buccal Mucosa. Pharmaceutics 2021, 13, 1361. [Google Scholar] [CrossRef]
- Morri, M.; Castellano, P.; Leonardi, D.; Vignaduzzo, S. First Development, Optimization, and Stability Control of a Pediatric Oral Atenolol Formulation. AAPS PharmSciTech 2018, 19, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, D.; Yatim, I.M.; Rahim, K.A.; Sanagi, M.M.; Ibrahim, W.A.W.; Aboul-Enein, H.Y. Comparison of HPLC and MEEKC for Miconazole Nitrate Determination in Pharmaceutical Formulation. Chromatographia 2013, 76, 1527–1536. [Google Scholar] [CrossRef]
- Tejada, G.; Barrera, M.G.; García, P.; Sortino, M.; Lamas, M.C.; Lassalle, V.; Alvarez, V.; Leonardi, D. Nanoparticulated Systems Based on Natural Polymers Loaded with Miconazole Nitrate and Lidocaine for the Treatment of Topical Candidiasis. AAPS PharmSciTech 2020, 21, 278. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Ikeda, N.; Tahara, K.; Takeuchi, H. Mechanical Characteristics of Orally Disintegrating Films: Comparison of Folding Endurance and Tensile Properties. Int. J. Pharm. 2020, 589, 119876. [Google Scholar] [CrossRef] [PubMed]
- Tejada, G.; Piccirilli, G.N.; Sortino, M.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Formulation and In-Vitro Efficacy of Antifungal Mucoadhesive Polymeric Matrices for the Delivery of Miconazole Nitrate. Mater. Sci. Eng. C 2017, 79, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Tejada, G.; Lamas, M.C.; Svetaz, L.; Salomón, C.J.; Alvarez, V.A.; Leonardi, D. Effect of Drug Incorporation Technique and Polymer Combination on the Performance of Biopolymeric Antifungal Buccal Films. Int. J. Pharm. 2018, 548, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Oza, N.A.; Makwana, A.; Gohil, T.A.; Shukla, P. Statistical Optimization of Miconazole Nitrate Microemulgel by Using 23 Full Factorial Desing. Int. J. Pharm. Sci. Drug Res. 2021, 13, 15–23. [Google Scholar] [CrossRef]
- Raj, V.; Prava, K.; Seru, G. RP-HPLC Method Development and Validation for the Simultaneous Determination of Clindamycin and Miconazole in Pharmaceutical Dosage Forms. Pharm. Methods 2014, 5, 56–60. [Google Scholar] [CrossRef]
- CLSI M44-A2; Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. Approved Guideline. 2nd ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; ISBN 1-56238-703-0.
- Calvo, N.L.; Svetaz, L.A.; Alvarez, V.A.; Quiroga, A.D.; Lamas, M.C.; Leonardi, D. Chitosan-Hydroxypropyl Methylcellulose Tioconazole Films: A Promising Alternative Dosage Form for the Treatment of Vaginal Candidiasis. Int. J. Pharm. 2019, 556, 181–191. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. Control of Encapsulation Efficiency and Initial Burst in Polymeric Microparticle Systems. Arch. Pharmacal Res. 2004, 27, 1–12. [Google Scholar] [CrossRef]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Characteristic of Gelatine, Carrageenan and Sodium Alginate Hydrosols Treated by Direct Electric Current. Polymers 2016, 8, 275. [Google Scholar] [CrossRef]
- Kalsoom Khan, A.; Saba, A.U.; Nawazish, S.; Akhtar, F.; Rashid, R.; Mir, S.; Nasir, B.; Iqbal, F.; Afzal, S.; Pervaiz, F.; et al. Carrageenan Dased Bionanocomposites as Drug Delivery Tool with Special Emphasis on the Influence of Ferromagnetic Nanoparticles. Oxid. Med. Cell. Longev. 2017, 2017, 8158315. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In Vitro Techniques to Evaluate Buccal Films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; Velasco, J.I.; de Oliveira, R.A. Active Edible Films Based on Arrowroot Starch with Microparticles of Blackberry Pulp Obtained by Freeze-Drying for Food Packaging. Polymers 2019, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tang, B.; Wu, P. Optimizing Polyamide Thin Film Composite Membrane Covalently Bonded with Modified Mesoporous Silica Nanoparticles. J. Membr. Sci. 2013, 428, 341–348. [Google Scholar] [CrossRef]
- Lu, R.; Sameen, D.E.; Qin, W.; Wu, D.; Dai, J.; Li, S.; Liu, Y. Development of Polylactic Acid Films with Selenium Microparticles and Its Application for Food Packaging. Coatings 2020, 10, 280. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Interaction of Cationic Antimicrobial (ε-Polylysine) with Food-Grade Biopolymers: Dextran, Chitosan, Carrageenan, Alginate, and Pectin. Food Res. Int. 2014, 64, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, R.J.; Rawn, J.D. Carboxylic Acid Derivatives. In Organic Chemistry; Academic Press: Cambridge, MA, USA, 2018; pp. 665–710. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and Characterization of Polyvinyl Alcohol-Gelatin Hydrogel Membranes for Biomedical Applications. AAPS PharmSciTech 2007, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; McConville, J.T. Manufacture and Characterization of Mucoadhesive Buccal Films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.J.; Shin, D.; Jo, K.; Lee, J. Thin Films as an Emerging Platform for Drug Delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Tedesco, M.P.; Monaco-Lourenço, C.A.; Carvalho, R.A. Gelatin/Hydroxypropyl Methylcellulose Matrices—Polymer Interactions Approach for Oral Disintegrating Films. Mater. Sci. Eng. C 2016, 69, 668–674. [Google Scholar] [CrossRef]
- Sakurai, K.; Maegawa, T.; Takahashi, T. Glass Transition Temperature of Chitosan and Miscibility of Chitosan/Poly(N-Vinyl Pyrrolidone) Blends. Polymer 2000, 41, 7051–7056. [Google Scholar] [CrossRef]
- Patel, N.; Lalwani, D.; Gollmer, S.; Injeti, E.; Sari, Y.; Nesamony, J. Development and Evaluation of a Calcium Alginate Based Oral Ceftriaxone Sodium Formulation. Prog. Biomater. 2016, 5, 117–133. [Google Scholar] [CrossRef]
- Rane, L.R.; Savadekar, N.R.; Kadam, P.G.; Mhaske, S.T. Preparation and Characterization of K-Carrageenan/Nanosilica Biocomposite Film. J. Mater. 2014, 2014, 736271. [Google Scholar] [CrossRef]
- Sadeghi, M. Synthesis of a Biocopolymer Carrageenan-g-Poly(AAm-Co-IA)/ Montmorilonite Superabsorbent Hydrogel Composite. Braz. J. Chem. Eng. 2012, 29, 295–305. [Google Scholar] [CrossRef]
- Bang, J.H.; Jang, Y.N.; Song, K.S.; Jeon, C.W.; Kim, W.; Lee, M.G.; Park, S.J. Effects of Sodium Laurylsulfate on Crystal Structure of Calcite Formed from Mixed Solutions. J. Colloid Interface Sci. 2011, 356, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Nedley, M.P.; Bhaduri, S.B.; Bredzinski, X.; Boddu, S.H.S. Masking the Bitter Taste of Injectable Lidocaine HCl Formulation for Dental Procedures. AAPS PharmSciTech 2015, 16, 455–465. [Google Scholar] [CrossRef]
- Tejada, G.; Lamas, M.C.; Sortino, M.; Alvarez, V.A.; Leonardi, D. Composite Microparticles Based on Natural Mucoadhesive Polymers with Promising Structural Properties to Protect and Improve the Antifungal Activity of Miconazole Nitrate. AAPS PharmSciTech 2018, 19, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Moseson, D.E.; Jordan, M.A.; Shah, D.D.; Corum, I.D.; Alvarenga, B.R.; Taylor, L.S. Application and Limitations of Thermogravimetric Analysis to Delineate the Hot Melt Extrusion Chemical Stability Processing Window. Int. J. Pharm. 2020, 590, 119916. [Google Scholar] [CrossRef]
- Umeyor, C.E.; Okoye, I.; Uronnachi, E.; Okeke, T.; Kenechukwu, F.; Attama, A. Repositioning Miconazole Nitrate for Malaria: Formulation of Sustained Release Nanostructured Lipid Carriers, Structure Characterization and in Vivo Antimalarial Evaluation. J. Drug Deliv. Sci. Technol. 2021, 61, 102125. [Google Scholar] [CrossRef]
- Zhou, G.; Dong, J.; Wang, Z.; Li, Z.; Li, Q.; Wang, B. Determination and Correlation of Solubility with Thermodynamic Analysis of Lidocaine Hydrochloride in Pure and Binary Solvents. J. Mol. Liq. 2018, 265, 442–449. [Google Scholar] [CrossRef]
- Trivino, A.; Gumireddy, A.; Meng, F.; Prasad, D.; Chauhan, H. Drug-Polymer Miscibility, Interactions, and Precipitation Inhibition Studies for the Development of Amorphous Solid Dispersions for the Poorly Soluble Anticancer Drug Flutamide. Drug Dev. Ind. Pharm. 2019, 45, 1277–1291. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving Drug Solubility for Oral Delivery Using Solid Dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Ribeiro, A.; Figueiras, A.; Santos, D.; Veiga, F. Preparation and Solid-State Characterization of Inclusion Complexes Formed between Miconazole and Methyl-β-Cyclodextrin. AAPS PharmSciTech 2008, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.U.; Nasir, F.; Maheen, S.; Shafqat, S.S.; Shah, S.; Khames, A.; Ghoneim, M.M.; Abbas, G.; Shabbir, S.; Abdelgawad, M.A.; et al. Antibacterial and Wound-Healing Activities of Statistically Optimized Nitrofurazone-and Lidocaine-Loaded Silica Microspheres by the Box–Behnken Design. Molecules 2022, 27, 2532. [Google Scholar] [CrossRef] [PubMed]








| Films Matrix Composition (Containing LDCH 2% w/w) | Microparticles Matrix Composition (Containing MN 5% w/w) | Films Loaded Microparticles Abbreviation |
|---|---|---|
| HPMC-GEL-LDCH | CH-ALG-MN | HG-CH-ALG |
| HPMC-GEL-LDCH | CH-κC-MN | HG-CH-κC |
| HPMC-GEL-LDCH | CH-λC-MN | HG-CH-λC |
| HPMC-GEL-LDCH | CH-SLS-MN | HG-CH-SLS |
| Film Formulation | Thickness (mm) | Folding Endurance | Tensile Strength (N) | Elongation (%) |
|---|---|---|---|---|
| HG-CH-ALG | 0.818 ± 0.085 | √ | 2.30 ± 0.57 | 12.11 ± 3.37 |
| HG-CH-κC | 0.696 ± 0.118 | √ | 2.20 ± 0.54 | 15.22 ± 5.07 |
| HG-CH-λC | 0.820 ± 0.075 | √ | 1.53 ± 0.33 | 14.97 ± 5.60 |
| HG-CH-SLS | 0.527 ± 0.076 | √ | 1.36 ± 0.48 | 19.04 ± 3.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada, G.; Calvo, N.L.; Morri, M.; Sortino, M.; Lamas, C.; Álvarez, V.A.; Leonardi, D. Miconazole Nitrate Microparticles in Lidocaine Loaded Films as a Treatment for Oropharyngeal Candidiasis. Materials 2023, 16, 3586. https://doi.org/10.3390/ma16093586
Tejada G, Calvo NL, Morri M, Sortino M, Lamas C, Álvarez VA, Leonardi D. Miconazole Nitrate Microparticles in Lidocaine Loaded Films as a Treatment for Oropharyngeal Candidiasis. Materials. 2023; 16(9):3586. https://doi.org/10.3390/ma16093586
Chicago/Turabian StyleTejada, Guillermo, Natalia L. Calvo, Mauro Morri, Maximiliano Sortino, Celina Lamas, Vera A. Álvarez, and Darío Leonardi. 2023. "Miconazole Nitrate Microparticles in Lidocaine Loaded Films as a Treatment for Oropharyngeal Candidiasis" Materials 16, no. 9: 3586. https://doi.org/10.3390/ma16093586
APA StyleTejada, G., Calvo, N. L., Morri, M., Sortino, M., Lamas, C., Álvarez, V. A., & Leonardi, D. (2023). Miconazole Nitrate Microparticles in Lidocaine Loaded Films as a Treatment for Oropharyngeal Candidiasis. Materials, 16(9), 3586. https://doi.org/10.3390/ma16093586