Enhanced Degradation of Ethylene in Thermo-Photocatalytic Process Using TiO2/Nickel Foam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Materials
2.1.1. Methods
- d = K × λ/(B−b) × cosθ, where:
- d—the average crystallite size;
- K—the shape factor;
- λ—the X-ray wavelength;
- B—the line width measured at FWHM, originating both from crystallite sizes and instrumental broadening;
- b—the line width originating solely from instrumental broadening;
- h—the reflex position.
2.1.2. Materials
3. Results
3.1. Physicochemical Properties of TiO2
3.2. Thermo-Photocatalytic Decomposition of Ethylene in the Presence of TiO2 and TiO2/Nickel Foam under UV-LED Light
3.3. Thermo-Photocatalytic Decomposition of Ethylene in the Presence of Radical Scavengers
3.4. FTIR Spectra of the Photocatalyst Surface Measured at the Condition of the Photocatalytic Process of Ethylene Decomposition
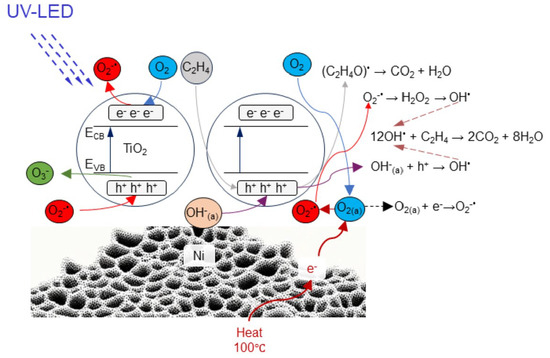
4. Discussion
- -
- under UV irradiation some hole and electrons species are formed in TiO2, which are utilized in the reactions:e− + O2 → O2•−h+ + −OH → •OH
- -
- hole traps (O•−) can react with adsorbed C2H4 to form (C2H4O)•, which undergoes further mineralisation to CO and CO2:O•− + C2H4 → (C2H4O)• → CO → CO2
- -
- hole traps can be also transformed to O3•− in the reaction with oxygen:O•− + O2 → O3•−
- -
- the presence of nickel foam increases separation of e−/h+ pairs in TiO2 giving higher yield in the photocatalytic reactions and also increases the electron traps in TiO2. Therefore in combination of TiO2 and nickel foam there is boosting of superoxide anionic radicals, which greatly contribute in the photocatalytic mineralisation of ethylene:O2•− → H2O2 → •OH12 •OH + C2H4 → 2CO2 + 8H2O
- -
- formed H2O upon C2H4 decomposition can be adsorbed on TiO2 surface and take parts in the further process of photocatalytic reactions.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pathak, N.; Caleb, O.J.; Geyer, M.; Herppich, W.B.; Rauh, C.; Mahajan, P.V. Photocatalytic and Photochemical Oxidation of Ethylene: Potential for Storage of Fresh Produce—A Review. Food Bioprocess Technol. 2017, 10, 982–1001. [Google Scholar] [CrossRef]
- Elsgaard, L. Ethylene Removal by a Biofilter with Immobilized Bacteria. Appl. Environ. Microbiol. 1998, 64, 4168–4173. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, H.Z.; Kheirkhah, B.; Kariminik, A. Ethylene Removal by Bio-filters in order to Increase Storage Life of Bananas. Int. J. Life Sci. 2015, 9, 62–65. [Google Scholar] [CrossRef]
- Smith, A.W.J.; Poulston, S.; Rowsell, L.; Terry, L.A.; Anderson, J.A. A New Palladium-Based Ethylene Scavenger to Control Ethylene-Induced Ripening of Climacteric Fruit. Platin. Met. Rev. 2009, 53, 112–122. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Bailén, G.; Serrano, M.; Guillén, F.; Valverde, J.M.; Zapata, P.; Castillo, S.; Valero, D. Tools to Maintain Postharvest Fruit and Vegetable Quality through the Inhibition of Ethylene Action: A Review. Crit. Rev. Food Sci. Nutr. 2007, 47, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Caleb, O.J.; Rauh, C.; Mahajan, P.V. Efficacy of photocatalysis and photolysis systems for the removal of ethylene under different storage conditions. Postharvest Biol. Technol. 2019, 147, 68–77. [Google Scholar] [CrossRef]
- Hussain, M.; Bensaid, S.; Geobaldo, F.; Saracco, G.; Russo, N. Photocatalytic Degradation of Ethylene Emitted by Fruits with TiO2 Nanoparticles. Ind. Eng. Chem. Res. 2011, 50, 2536–2543. [Google Scholar] [CrossRef]
- Parka, D.R.; Zhang, J.; Ikeueb, K.; Yamashitab, H.; Anpo, M. Photocatalytic Oxidation of Ethylene to CO2 and H2O on Ultrafine Powdered TiO2 Photocatalysts in the Presence of O2 and H2O. J. Catal. 1999, 185, 114–119. [Google Scholar] [CrossRef]
- Kumar, S.; Fedorov, A.G.; Gole, J.L. Photodegradation of ethylene using visible light responsive surfaces prepared from titania nanoparticle slurries. Appl. Catal. B Environ. 2005, 57, 93–107. [Google Scholar] [CrossRef]
- Muramoto, Y.; Kimura, M.; Nouda, S. Development and future of ultraviolet light-emitting diodes: UV-LED will replace the UV lamp. Semicond. Sci. Technol. 2014, 29, 084004. [Google Scholar] [CrossRef]
- Fonseca, J.d.M.; Miranda, S.M.; Monteiro, J.P.; Moreira, R.d.F.P.M.; Valencia, G.A.; Monteiro, A.R.; Vilar, V.J. Ethylene photocatalytic degradation using TiO2 immobilized in a NETmix mili-photoreactor. J. Environ. Chem. Eng. 2023, 11, 110976. [Google Scholar] [CrossRef]
- Shi, G.; Mahmood, A.; Lu, G.; Wang, X.; Tong, S.; Ge, M.; Xie, X.; Sun, J. Adsorption and Photodegradation of Acetaldehyde and Ethylene on TiO2 (001) Surface: Experimental and First Principle Studies. Catal. Lett. 2019, 149, 2728–2738. [Google Scholar] [CrossRef]
- Rychtowski, P.; Tryba, B.; Skrzypska, A.; Felczak, P.; Sreńscek-Nazzal, J.; Wróbel, R.J.; Nishiguchi, H.; Toyoda, M. Role of the Hydroxyl Groups Coordinated toTiO2 Surface on the Photocatalytic Decomposition of Ethylene at Different Ambient Conditions. Catalysts 2022, 12, 386. [Google Scholar] [CrossRef]
- Xia, J.; Dong, L.; Song, H.; Yang, J.; Zhu, X. Preparation of doped TiO2 nanomaterials and their applications in photocatalysis. Bull. Mater. Sci. 2023, 46, 13. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Varma, S.; Tripathi, A.K.; Bharadwaj, S.R.; Tyagi, A.K. Mechanistic Insight by in Situ FTIR for the Gas Phase Photo-oxidation of Ethylene by V-Doped Titania and Nano Titania. J. Phys. Chem. B 2009, 113, 5917–5928. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Xie, X.; Wang, X.; Mahmood, A.; Tong, S.; Ge, M.; Sun, J. Defect Chemistry of Er3+-Doped TiO2 and Its Photocatalytic Activity for the Degradation of Flowing Gas-Phase VOCs. J. Phys. Chem. C 2019, 123, 12321–12334. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, S.; Chen, X.; Song, X.; Li, L.; Huang, X. Photocatalytic degradation of ethylene using titanium dioxide nanotube arrays with Ag and reduced graphene oxide irradiated by γ-ray radiolysis. Appl. Catal. B Environ. 2017, 203, 673–683. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Weng, C.-H.; Srivastav, A.L.; Lin, Y.-T.; Tzeng, J.-H. Facile Synthesis and Characterization of N-Doped TiO2Photocatalyst and Its Visible-Light Activity for Photo-Oxidation of Ethylene. J. Nanomater. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Chen, F.-Y. Key operating parameters affecting photocatalytic activity of visible-light-induced C-doped TiO2 catalyst for ethylene oxidation. Chem. Eng. J. 2014, 248, 175–183. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kang, H.-J. Aluminum sheet-based S-doped TiO2 for photocatalytic decomposition of toxic organic vapors. Chin. J. Catal. 2014, 35, 1189–1195. [Google Scholar] [CrossRef]
- Yang, H.; Yang, B.; Chen, W.; Yang, J. Preparation and Photocatalytic Activities of TiO2-Based Composite Catalysts. Catalysts 2022, 12, 1263. [Google Scholar] [CrossRef]
- Kajita, S.; Miyaguchi, K.; Tanaka, H.; Yasunaga, E.; Yoshida, T.; Ohno, N. Enhanced photocatalytic ethylene decomposition with anatase-rutile mixed nanostructures formed by He plasma treatment. J. Photochem. Photobiol. A Chem. 2021, 418, 113420. [Google Scholar] [CrossRef]
- Mei, X.; Yuan, H.; Li, C. Study on the MOF Frame Pt-TiO2 Hybrid Photocatalyst and Its Photocatalytic Performance. Sustainability 2023, 15, 1403. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Song, X.; Luo, S.; Ye, S.; Situ, W. Photocatalytic degradation of ethylene in cold storage using the nanocomposite photocatalyst MIL101(Fe)-TiO2-rGO. Chem. Eng. J. 2021, 424, 130407. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Valdés, H.; Mangalaraja, R.V.; Sathishkumar, P.; Murugesan, S. Graphene modified “black {0 0 1}TiO2” nanosheets for photocatalytic oxidation of ethylene: The implications of chemical surface characteristics in the reaction mechanism. Sep. Purif. Technol. 2022, 292, 121008. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, W.; Duan, J.; Fu, L.; Ma, L.; Yang, Z. Immobilized Ag3PO4/GO on 3D nickel foam and its photocatalytic degradation of norfloxacin antibiotic under visible light. RSC Adv. 2020, 10, 4427–4435. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, F.; Chang, X.; He, D. Comparison of Nickel Foam/Ag-Supported ZnO, TiO2, and WO3for Toluene Photodegradation. Mater. Manuf. Process. 2014, 29, 789–794. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Shi, J.; Hu, H.; Shangguan, W. Design consideration of photocatalytic oxidation reactors using TiO2-coated foam nickels for degrading indoor gaseous formaldehyde. Catal. Today 2007, 126, 359–368. [Google Scholar] [CrossRef]
- Hu, H.; Xiao, W.; Yuan, J.; Shi, J.; Shangguan, W. TiO2/SiO2Composite Films Immobilized on Foam Nickel Substrate for the Photocatalytic Degradation of Gaseous Acetaldehyde. Int. J. Photoenergy 2008, 2008, 679421. [Google Scholar] [CrossRef]
- Tryba, B.; Miądlicki, P.; Rychtowski, P.; Trzeciak, M.; Wróbel, R.J. The Superiority of TiO2 Supported on Nickel Foam over Ni-Doped TiO2 in the Photothermal Decomposition of Acetaldehyde. Materials 2023, 16, 5241. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xiao, W.; Yuan, J.; Shi, J.; He, D.; Shangguan, W. High photocatalytic activity and stability for decomposition of gaseous acetaldehyde on TiO2/Al2O3 composite films coated on foam nickel substrates by sol-gel processes. J. Sol.-Gel Sci. Technol. 2008, 45, 1–8. [Google Scholar] [CrossRef]
- Tryba, B.; Orlikowski, J.; Wróbel, R.J.; Przepiórski, J.; Morawski, A.W. Preparation and Characterization of Rutile-Type TiO2 Doped with Cu. J. Mater. Eng. Perform. 2015, 24, 1243–1252. [Google Scholar] [CrossRef]
- Zeng, Q.; Xie, X.; Wang, X.; Wang, Y.; Lu, G.; Pui, D.Y.; Sun, J. Enhanced photocatalytic performance of Ag@TiO2 for the gaseous acetaldehyde photodegradation under fluorescent lamp. Chem. Eng. J. 2018, 341, 83–92. [Google Scholar] [CrossRef]
- Fu, X.; Clark, L.A.; Zeltner, W.A.; Anderson, M.A. Effects of reaction temperature and water vapor content on the heterogeneous photocatalytic oxidation of ethylene. J. Photochem. Photobiol. A Chem. 1996, 97, 181–186. [Google Scholar] [CrossRef]
- Mphuthi, L.E.; Maseme, M.R.; Langner, E.H.G. Ti(IV)-Exchanged Nano-ZIF-8 and Nano-ZIF-67 for Enhanced Photocatalytic Oxidation of Hydroquinone. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2664–2678. [Google Scholar] [CrossRef]
- Ammawath, W.; Man, Y.C.; Baharin, B.; Rahman, R.A. A new method for determination of tert-butylhydroquinone (tbhq) in rbd palm olein with ftir spectroscopy. J. Food Lipids 2004, 11, 266–277. [Google Scholar] [CrossRef]
- Fónagy, O.; Szabó-Bárdos, E.; Horváth, O. 1,4-Benzoquinone and 1,4-hydroquinone based determination of electron and superoxide radical formed in heterogeneous photocatalytic systems. J. Photochem. Photobiol. A Chem. 2021, 407, 113057. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzeciak, M.; Miądlicki, P.; Tryba, B. Enhanced Degradation of Ethylene in Thermo-Photocatalytic Process Using TiO2/Nickel Foam. Materials 2024, 17, 267. https://doi.org/10.3390/ma17010267
Trzeciak M, Miądlicki P, Tryba B. Enhanced Degradation of Ethylene in Thermo-Photocatalytic Process Using TiO2/Nickel Foam. Materials. 2024; 17(1):267. https://doi.org/10.3390/ma17010267
Chicago/Turabian StyleTrzeciak, Maciej, Piotr Miądlicki, and Beata Tryba. 2024. "Enhanced Degradation of Ethylene in Thermo-Photocatalytic Process Using TiO2/Nickel Foam" Materials 17, no. 1: 267. https://doi.org/10.3390/ma17010267
APA StyleTrzeciak, M., Miądlicki, P., & Tryba, B. (2024). Enhanced Degradation of Ethylene in Thermo-Photocatalytic Process Using TiO2/Nickel Foam. Materials, 17(1), 267. https://doi.org/10.3390/ma17010267