Catalytic Oxidation of Chlorobenzene over HSiW/CeO2 as a Co-Benefit of NOx Reduction: Remarkable Inhibition of Chlorobenzene Oxidation by NH3
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.2. Catalytic Performance Evaluation
2.3. Catalyst Characterization
3. Results and Discussion
3.1. Performances for NOx Reduction and CB Oxidation
3.1.1. Activity and Product Selectivity
3.1.2. Long-Term Stability
3.2. Characterization
3.2.1. XRD and BET Surface Area
3.2.2. XPS
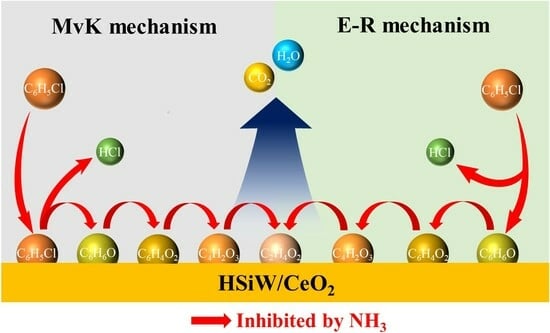
3.3. Mechanism of CB Oxidation
3.4. Inhibition Mechanism of NH3 on CB Oxidation
3.5. CB Oxidation under a Low GHSV of Normal SCR Condition
3.6. Significance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brummer, V.; Jecha, D.; Skryja, P.; Stehlik, P. Pilot VOC emissions mitigation by catalytic oxidation over commercial noble metal catalysts for cleaner production. Clean Technol. Environ. Policy 2023, 25, 2249–2262. [Google Scholar] [CrossRef]
- Cech, P.; Stádník, J. VOC emissions from natural upholstery leathers. Pol. J. Environ. Stud. 2021, 30, 4945–4955. [Google Scholar] [CrossRef] [PubMed]
- Monard, C.; Caudal, J.P.; Cluzeau, D.; Le Garrec, J.L.; Hellequin, E.; Hoeffner, K.; Humbert, G.; Jung, V.C.; Le Lann, C.; Nicolai, A. Short-term temporal dynamics of VOC emissions by soil systems in different biotopes. Front. Environ. Sci. 2021, 9, 650701. [Google Scholar] [CrossRef]
- Halawy, S.A.; Osman, A.I.; Mehta, N.; Abdelkader, A.; Vo, D.V.N.; Rooney, D.W. Adsorptive removal of some Cl-VOC’s as dangerous environmental pollutants using feather-like γ-Al2O3 derived from aluminium waste with life cycle analysis. Chemosphere 2022, 295, 133795. [Google Scholar] [CrossRef] [PubMed]
- Le Breton, M.; Hallquist, Å.; Pathak, R.K.; Simpson, D.; Wang, Y.J.; Johansson, J.; Zheng, J.; Yang, Y.D.; Shang, D.J.; Wang, H.C.; et al. Chlorine oxidation of VOCs at a semi-rural site in Beijing: Significant chlorine liberation from ClNO2 and subsequent gas- and particle-phase Cl-VOC production. Atmos. Chem. Phys. 2018, 18, 13013–13030. [Google Scholar] [CrossRef]
- Aathira, B.; Deepika, S.; Sounak, R.; Singh, S.A. Technological solutions for NOx, SOx, and VOC abatement: Recent breakthroughs and future directions. Environ. Sci. Pollut. Res. 2023, 30, 91501–91533. [Google Scholar]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Gallastegi-Villa, M.; Romero-Sáez, M.; Aranzabal, A.; González-Marcos, J.A.; González-Velasco, J.R. Strategies to enhance the stability of h-bea zeolite in the catalytic oxidation of Cl-VOCs: 1,2-Dichloroethane. Catal. Today 2013, 213, 192–197. [Google Scholar] [CrossRef]
- Delaigle, R.; Eloy, P.; Gaigneaux, E.M. Influence of the impregnation order on the synergy between Ag and V2O5/TiO2 catalysts in the total oxidation of Cl-aromatic VOC. Catal. Today 2012, 192, 2–9. [Google Scholar] [CrossRef]
- Song, Z.; Peng, Y.; Zhao, X.; Liu, H.; Gao, C.; Si, W.; Li, J. Roles of Ru on the V2O5-WO3/TiO2 catalyst for the simultaneous purification of NOx and chlorobenzene: A dechlorination promoter and a redox Inductor. ACS Catal. 2022, 12, 11505–11517. [Google Scholar] [CrossRef]
- Yuan, X.; Peng, Y.; Zhu, X.; Wang, H.; Song, Z.; Si, W.; Wang, Y.; Li, J. Anti-poisoning mechanisms of Sb on vanadia-based catalysts for NOx and chlorobenzene multi-pollutant control. Environ. Sci. Technol. 2023, 57, 10211–10220. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.K.; Chandel, V.S.; Singh, O.P.; Alam, N. Synthesis of CeO2 and Zr-Doped CeO2 (Ce1-xZrxO2) catalyst by green synthesis for soot oxidation activity. Arab. J. Sci. Eng. 2023, 48, 771–777. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Li, Q.; Wang, Z.; Zhang, Z.; Zheng, L. Ce–Ti amorphous oxides for selective catalytic reduction of NO with NH3: Confirmation of Ce–O–Ti active sites. Environ. Sci. Technol. 2012, 46, 9600–9605. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, J.; Chen, L.; Chen, J.; Han, J.; Zhang, H.; Han, W. Alkali metal poisoning of a CeO2-WO3 catalyst used in the selective catalytic reduction of NOx with NH3: An experimental and theoretical study. Environ. Sci. Technol. 2012, 46, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Cai, S.; Chen, J.; Yan, D.; Jiang, M.; Chen, J.; Jia, H. Tuning the degradation activity and pathways of chlorinated organic pollutants over CeO2 catalyst with acid sites: Synergistic effect of Lewis and Brønsted acid sites. Catal. Sci. Technol. 2021, 11, 4581–4595. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, W.; Yin, R.; Sun, P.; Lu, Y.; Wu, Z.; Weng, X. The role of surface sulfation in mediating the acidity and oxidation ability of nickel modified ceria catalyst for the catalytic elimination of chlorinated organics. J. Colloid Interf. Sci. 2020, 574, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Jin, K.; Mei, J.; Su, G.Y.; Ma, L.; Yang, S.J. CeO2 grafted with different heteropoly acids for selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2020, 382, 121032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, Y.; Song, Z.; Liu, W.; Gao, C.; Luo, J. Silicotungstic acid modified CeO2 catalyst with high stability for the catalytic combustion of chlorobenzene. Chemosphere 2021, 263, 128129. [Google Scholar] [CrossRef]
- Koller, V.; Sack, C.; Lustemberg, P.; Ganduglia-Pirovano, M.V.; Over, H. Dynamic response of oxygen vacancies in the deacon reaction over reduced single crystalline CeO2-x (111) surfaces. J. Phys. Chem. C 2022, 126, 13202–13212. [Google Scholar] [CrossRef]
- Koller, V.; Lustemberg, P.G.; Spriewald-Luciano, A.; Gericke, S.M.; Larsson, A.; Sack, C.; Preobrajenski, A.; Lundgren, E.; Ganduglia-Pirovano, M.V.; Over, H. Critical step in the HCl oxidation reaction over single-crystalline CeO2-x (111): Peroxo-induced site change of strongly adsorbed surface chlorine. ACS Catal. 2023, 13, 12994–13007. [Google Scholar] [CrossRef]
- Hunpratub, S.; Chullaphan, T.; Chumpolkulwong, S.; Chanlek, N.; Phokha, S. Characterization and electrochemical properties of carbon/CeO2 composites prepared using a hydrothermal method. Mater. Chem. Phys. 2023, 303, 127820. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; López-Rodríguez, S.; Villar-García, I.J.; Pérez-Dieste, V.; Bueno-López, A.; González-Velasco, J.R. Molecular elucidation of CO2 methanation over a highly active, selective and stable LaNiO3/CeO2-derived catalyst by in situ FTIR and NAP-XPS. Appl. Catal. B Environ. 2024, 342, 123367. [Google Scholar] [CrossRef]
- Kumar, V.B.; Pulidindi, I.N.; Gedanken, A. Selective conversion of starch to glucose using carbon based solid acid catalyst. Renew. Energy 2015, 78, 141–145. [Google Scholar] [CrossRef]
- Scirè, S.; Riccobene, P.M.; Crisafulli, C. Ceria supported group IB metal catalysts for the combustion of volatile organic compounds and the preferential oxidation of CO. Appl. Catal. B Environ. 2010, 101, 109–117. [Google Scholar] [CrossRef]
- Aranzabal, A.; Ayastuy-Arizti, J.L.; González-Marcos, J.A.; González-Velasco, J.R. The reaction pathway and kinetic mechanism of the catalytic oxidation of gaseous lean TCE on Pd/alumina catalysts. J. Catal. 2003, 214, 130–135. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.Q.; Lv, X.L.; Huang, G.X.; Xu, W.J.; Li, X.L.; Jia, H.P. Pt/CeO2 coated with polyoxometallate chainmail to regulate oxidation of chlorobenzene without hazardous by-products. J. Hazard. Mater. 2023, 441, 129925. [Google Scholar] [CrossRef]
- Lomnicki, S.; Lichtenberger, J.; Xu, Z.; Waters, M.; Kosman, J.; Amiridis, M.D. Catalytic oxidation of 2,4,6-trichlorophenol over vanadia/titania-based catalysts. Appl. Catal. B Environ. 2003, 46, 105–119. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, J.; Shi, W.; Wang, J.; Zhu, T.; Chen, Y. Catalytic oxidation of benzene over ruthenium-cobalt bimetallic catalysts and study of its mechanism. Catal. Sci. Technol. 2017, 7, 213–221. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, X.; Zhu, T.; Guo, Y.; Qi, H. Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts: The effects of chlorine substituents. Catal. Today 2015, 241, 92–99. [Google Scholar] [CrossRef]
- Hauchecorne, B.; Terrens, D.; Verbruggen, S.; Martens, J.A.; Van Langenhove, H.; Demeestere, K.; Lenaerts, S. Elucidating the photocatalytic degradation pathway of acetaldehyde: An FTIR in situ study under atmospheric conditions. Appl. Catal. B Environ. 2011, 106, 630–638. [Google Scholar] [CrossRef]
- Liang, W.; Zhu, Y.; Ren, S.; Shi, X. Enhanced catalytic elimination of chlorobenzene over Ru/TiO2 modified with SnO2—Synergistic performance of oxidation and acidity. Chem. Phys. 2023, 566, 111787. [Google Scholar] [CrossRef]
- Hetrick, C.E.; Lichtenberger, J.; Amiridis, M.D. Catalytic oxidation of chlorophenol over V2O5/TiO2 catalysts. Appl. Catal. B Environ. 2008, 77, 255–263. [Google Scholar] [CrossRef]
- Vainio, E.; Yrjas, P.; Hupa, L.; Hupa, M. Cold-end corrosion caused by hygroscopic ammonium chloride in thermal conversion of biomass and waste. Fuel 2023, 346, 128061. [Google Scholar] [CrossRef]
- Jabłońska, M.; Robles, A.M. A comparative mini-review on transition metal oxides applied for the selective catalytic ammonia oxidation (NH3-SCO). Materials 2022, 15, 4770. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Lee, M.J.; Kim, W.G.; Kim, S.J.; Jeong, B.; Ye, B.; Kim, H.D. Oxidation of CO and slipped NH3 over a WS2-Loaded Pt/TiO2 catalyst with an extended reaction temperature range. Mater. Chem. Phys. 2023, 309, 128341. [Google Scholar] [CrossRef]
- Gouveia, J.D.; Gomes, J.R.B. Effect of the surface termination on the adsorption of flue gas by the titanium carbide MXene. Mater. Today Chem. 2023, 29, 101441. [Google Scholar] [CrossRef]
- Jacobs, J.H.; Chou, N.Y.; Lesage, K.L.; Xiao, Y.; Hill, J.M.; Marriott, R.A. Investigating activated carbons for SO2 adsorption in wet flue gas. Fuel 2023, 353, 129239. [Google Scholar] [CrossRef]













| Temperature/°C | /μmol g−1 min−1 | |||
|---|---|---|---|---|
| kE-R | kMvK | R2 | ||
| HSiW/CeO2 | 250 | 0.008 | 2.77 | 0.998 |
| 300 | 0.021 | 3.89 | 0.995 | |
| 350 | 0.060 | 4.07 | 0.999 | |
| 400 | 0.132 | 4.63 | 0.996 | |
| 450 | 0.170 | 4.79 | 0.998 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Jiang, K.; Shen, Q.; Xie, L.; Mei, J.; Yang, S. Catalytic Oxidation of Chlorobenzene over HSiW/CeO2 as a Co-Benefit of NOx Reduction: Remarkable Inhibition of Chlorobenzene Oxidation by NH3. Materials 2024, 17, 828. https://doi.org/10.3390/ma17040828
Dong L, Jiang K, Shen Q, Xie L, Mei J, Yang S. Catalytic Oxidation of Chlorobenzene over HSiW/CeO2 as a Co-Benefit of NOx Reduction: Remarkable Inhibition of Chlorobenzene Oxidation by NH3. Materials. 2024; 17(4):828. https://doi.org/10.3390/ma17040828
Chicago/Turabian StyleDong, Leyuan, Keyu Jiang, Qi Shen, Lijuan Xie, Jian Mei, and Shijian Yang. 2024. "Catalytic Oxidation of Chlorobenzene over HSiW/CeO2 as a Co-Benefit of NOx Reduction: Remarkable Inhibition of Chlorobenzene Oxidation by NH3" Materials 17, no. 4: 828. https://doi.org/10.3390/ma17040828
APA StyleDong, L., Jiang, K., Shen, Q., Xie, L., Mei, J., & Yang, S. (2024). Catalytic Oxidation of Chlorobenzene over HSiW/CeO2 as a Co-Benefit of NOx Reduction: Remarkable Inhibition of Chlorobenzene Oxidation by NH3. Materials, 17(4), 828. https://doi.org/10.3390/ma17040828