Development of a Resveratrol Nanoformulation for the Treatment of Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
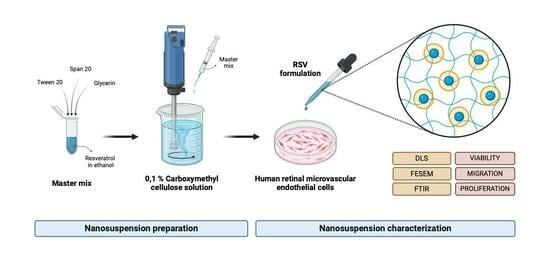
2.2. RSV-NS Preparation
2.3. RVS-NS Physicochemical Characterization
2.4. RSV-NS Biological Characterization
2.4.1. Cell Cytotoxicity Assay
2.4.2. Cell Migration Assay
2.4.3. Cell Proliferation Assay
2.5. Statistical Analysis
3. Results
3.1. RSV-NS Characterization
3.2. Cell Cytotoxicity
3.3. Cell Migration and Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, L.; Qiao, H. Diabetic Retinopathy Classification Using a Novel DAG Network Based on Multi-Feature of Fundus Images. Biomed. Signal Process. Control 2022, 77, 103810. [Google Scholar] [CrossRef]
- Kovoor, E.; Chauhan, S.K.; Hajrasouliha, A. Role of Inflammatory Cells in Pathophysiology and Management of Diabetic Retinopathy. Surv. Ophthalmol. 2022, 67, 1563–1573. [Google Scholar] [CrossRef]
- López-Malo, D.; Villarón-Casares, C.A.; Alarcón-Jiménez, J.; Miranda, M.; Díaz-Llopis, M.; Romero, F.J.; Villar, V.M. Curcumin as a Therapeutic Option in Retinal Diseases. Antioxidants 2020, 9, 48. [Google Scholar] [CrossRef]
- Li, R.; Chen, L.; Yao, G.-M.; Yan, H.-L.; Wang, L. Effects of Quercetin on Diabetic Retinopathy and Its Association with NLRP3 Inflammasome and Autophagy. Int. J. Ophthalmol. 2021, 14, 42–49. [Google Scholar] [CrossRef]
- Merrigan, S.L.; Park, B.; Ali, Z.; Jensen, L.D.; Corson, T.W.; Kennedy, B.N. Calcitriol and Non-Calcemic Vitamin D Analogue, 22-Oxacalcitriol, Attenuate Developmental and Pathological Choroidal Vasculature Angiogenesis Ex Vivo and In Vivo. Oncotarget 2020, 11, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid. Med. Cell. Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Li, X.-H.; Chen, M.; Luo, J. Intravitreal Injection of Resveratrol Inhibits Laser-Induced Murine Choroidal Neovascularization. Int. J. Ophthalmol. 2020, 13, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant Activity and Mechanism of Resveratrol and Polydatin Isolated from Mulberry (Morus alba L.). Molecules 2021, 26, 7574. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Pecora, T.M.G.; Cutuli, G.G.; Catalfo, A.; De Guidi, G.; Ruozi, B.; Tosi, G.; Cianciolo, S.; Musumeci, T. Antioxidant Activity and Photostability Assessment of Trans-Resveratrol Acrylate Microspheres. Pharm. Dev. Technol. 2019, 24, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients 2021, 13, 3095. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Li, R.; Yan, M. New Resveratrol Micelle Formulation for Ocular Delivery: Characterization and in Vitro/in Vivo Evaluation. Drug Dev. Ind. Pharm. 2020, 46, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; García Liñares, G.; Acebedo, S.L.; Castañeda Cataña, M.A.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol Encapsulation in High Molecular Weight Chitosan-Based Nanogels for Applications in Ocular Treatments: Impact on Human ARPE-19 Culture Cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Lopez, M.; Sparacino, C.; Quelle-Regaldie, A.; Sánchez, L.; Candal, E.; Barreiro-Iglesias, A.; Huete-Toral, F.; Carracedo, G.; Otero, A.; Concheiro, A.; et al. Pluronic®/Casein Micelles for Ophthalmic Delivery of Resveratrol: In Vitro, Ex Vivo, and in Vivo Tests. Int. J. Pharm. 2022, 628, 122281. [Google Scholar] [CrossRef]
- Zingale, E.; Bonaccorso, A.; D’Amico, A.G.; Lombardo, R.; D’Agata, V.; Rautio, J.; Pignatello, R. Formulating Resveratrol and Melatonin Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Ocular Administration Using Design of Experiments. Pharmaceutics 2024, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End. Polymers 2021, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Shaabani, A. Carboxymethyl Cellulose-Based Oral Delivery Systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Buljeta, I.; Šimunović, J.; Kopjar, M. Carboxymethylcellulose Hydrogels: Effect of Its Different Amount on Preservation of Tart Cherry Anthocyanins and Polyphenols. Curr. Plant Biol. 2021, 28, 100222. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Omerović, N.; Vranić, E. Application of Nanoparticles in Ocular Drug Delivery Systems. Health Technol. 2020, 10, 61–78. [Google Scholar] [CrossRef]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G.P.; Valapala, M.; Kerur, N.; Passaglia, C.L. Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent Developments in Functionalized Polymer Nanoparticles for Efficient Drug Delivery System. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-Based Ocular Drug Delivery Systems: Recent Advances and Future Prospects. J. Nanobiotechnol. 2023, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Aldeeb, M.; Wilar, G.; Suhandi, C.; Elamin, K.; Wathoni, N. Nanosuspension-Based Drug Delivery Systems for Topical Applications. Int. J. Nanomed. 2024, 19, 825–844. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Xu, L.; Wang, Q.; Wu, Z.; Zhang, H.; Gan, L. Cyclosporine A Nanosuspensions for Ophthalmic Delivery: A Comparative Study between Cationic Nanoparticles and Drug-Core Mucus Penetrating Nanoparticles. Mol. Pharm. 2021, 18, 4290–4298. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Vora, L.K.; Mishra, D.; Adrianto, M.F.; Gade, S.; Paredes, A.J.; Donnelly, R.F.; Singh, T.R.R. Nanosuspension-Loaded Dissolving Bilayer Microneedles for Hydrophobic Drug Delivery to the Posterior Segment of the Eye. Biomater. Adv. 2022, 137, 212767. [Google Scholar] [CrossRef] [PubMed]
- Kuk, D.-H.; Ha, E.-S.; Ha, D.-H.; Sim, W.-Y.; Lee, S.-K.; Jeong, J.-S.; Kim, J.-S.; Baek, I.; Park, H.; Choi, D.H.; et al. Development of a Resveratrol Nanosuspension Using the Antisolvent Precipitation Method without Solvent Removal, Based on a Quality by Design (QbD) Approach. Pharmaceutics 2019, 11, 688. [Google Scholar] [CrossRef]
- Keck, C.M.; Kobierski, S.; Ofori-Kwakye, K.; Müller, R.H. Resveratrol Nanosuspensions for Dermal Application-Production, Characterization, and Physical Stability. Pharmazie 2009, 64, 741–747. [Google Scholar] [CrossRef]
- Hao, J.; Zhao, J.; Zhang, S.; Tong, T.; Zhuang, Q.; Jin, K.; Chen, W.; Tang, H. Fabrication of an Ionic-Sensitive in Situ Gel Loaded with Resveratrol Nanosuspensions Intended for Direct Nose-to-Brain Delivery. Colloids Surf. B Biointerfaces 2016, 147, 376–386. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.; Sun, Z.; Shen, X.; Li, L.; Jin, L.; Mao, S. Influence of Stabilizer Type and Concentration on the Lung Deposition and Retention of Resveratrol Nanosuspension-in-Microparticles. Int. J. Pharm. 2019, 569, 118562. [Google Scholar] [CrossRef]
- Hao, J.; Gao, Y.; Zhao, J.; Zhang, J.; Li, Q.; Zhao, Z.; Liu, J. Preparation and Optimization of Resveratrol Nanosuspensions by Antisolvent Precipitation Using Box-Behnken Design. AAPS PharmSciTech 2015, 16, 118–128. [Google Scholar] [CrossRef]
- Whitmore, H.A.B.; Amarnani, D.; O’Hare, M.; Delgado-Tirado, S.; Gonzalez-Buendia, L.; An, M.; Pedron, J.; Bushweller, J.H.; Arboleda-Velasquez, J.F.; Kim, L.A. TNF-α Signaling Regulates RUNX1 Function in Endothelial Cells. FASEB J. 2021, 35, e21155. [Google Scholar] [CrossRef] [PubMed]
- Amanat, S.; Taymouri, S.; Varshosaz, J.; Minaiyan, M.; Talebi, A. Carboxymethyl Cellulose-Based Wafer Enriched with Resveratrol-Loaded Nanoparticles for Enhanced Wound Healing. Drug Deliv. Transl. Res. 2020, 10, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of Reaction Conditions for Preparing Carboxymethyl Cellulose from Sago Waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Al-Jumaily, E.F.; Hamid, G.S.; Ali, K.F. Synthesis and Total Phenol Content of New Resveratrol Derivative. Open J. Adv. Drug Deliv. 2014, 2, 320–329. [Google Scholar]
- Kumpugdee-Vollrath, M.; Ibold, Y.; Sriamornsak, P. Solid State Characterization of Trans Resveratrol Complexes with Different Cyclodextrins. J. Asian Assoc. Sch. Pharm. 2012, 1, 125–136. [Google Scholar]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol Encapsulation: Designing Delivery Systems to Overcome Solubility, Stability and Bioavailability Issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, F.; Guo, Z.; Zhao, Y.L. Preparation and Characterization of Resveratrol/Hydroxypropyl-β-Cyclodextrin Inclusion Complex Using Supercritical Antisolvent Technology. J. Food Process Eng. 2012, 35, 677–686. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of Resveratrol Coated Gold Nanoparticles and Investigation of Their Effect on Diabetic Retinopathy in Streptozotocin Induced Diabetic Rats. J. Photochem. Photobiol. B Biol. 2019, 195, 51–57. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays; Gilbert, D.F., Friedrich, O., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1601, pp. 1–17. ISBN 978-1-4939-6959-3. [Google Scholar]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Steinbrecht, S.; König, R.; Schmidtke, K.-U.; Herzog, N.; Scheibner, K.; Krüger-Genge, A.; Jung, F.; Kammerer, S.; Küpper, J.-H. Metabolic Activity Testing Can Underestimate Acute Drug Cytotoxicity as Revealed by HepG2 Cell Clones Overexpressing Cytochrome P450 2C19 and 3A4. Toxicology 2019, 412, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Cell Counting Kit 8 (WTS-8/CCK8) (ab228554). Available online: https://www.abcam.com/products/assay-kits/cell-counting-kit-8-wst-8--cck8-ab228554.html (accessed on 20 February 2023).
- Toro, M.D.; Nowomiejska, K.; Avitabile, T.; Rejdak, R.; Tripodi, S.; Porta, A.; Reibaldi, M.; Figus, M.; Posarelli, C.; Fiedorowicz, M. Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 3503. [Google Scholar] [CrossRef]
- Chung, J.H.; Lee, J.-S.; Lee, H.G. Resveratrol-Loaded Chitosan–γ-Poly(Glutamic Acid) Nanoparticles: Optimization, Solubility, UV Stability, and Cellular Antioxidant Activity. Colloids Surf. B Biointerfaces 2020, 186, 110702. [Google Scholar] [CrossRef]
- Robertson, I.; Wai Hau, T.; Sami, F.; Sajid Ali, M.; Badgujar, V.; Murtuja, S.; Saquib Hasnain, M.; Khan, A.; Majeed, S.; Tahir Ansari, M. The Science of Resveratrol, Formulation, Pharmacokinetic Barriers and Its Chemotherapeutic Potential. Int. J. Pharm. 2022, 618, 121605. [Google Scholar] [CrossRef]
- Hanagandi, V.; Sidagouda Patil, A.; Masareddy, R.S.; Dandagi, P.M.; Bolmal, U.B. Development and Evaluation of Nanosuspension Incorporated In Situ Gel of Brimonidine Tartarate for Ocular Drug Delivery. Indian J. Pharm. Educ. Res. 2022, 56, 94–102. [Google Scholar] [CrossRef]
- Khare, P.; Chogale, M.M.; Kakade, P.; Patravale, V.B. Gellan Gum–Based in Situ Gelling Ophthalmic Nanosuspension of Posaconazole. Drug Deliv. Transl. Res. 2022, 12, 2920–2935. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; ISBN 978-0-85369-792-3. [Google Scholar]
- Soroushnia, A.; Ganji, F.; Vasheghani-Farahani, E.; Mobedi, H. Effect of Combined Stabilizers on Midazolam Nanosuspension Properties. Iran. Polym. J. 2022, 31, 215–222. [Google Scholar] [CrossRef]
- Jeong, H.; Samdani, K.J.; Yoo, D.H.; Lee, D.W.; Kim, N.H.; Yoo, I.-S.; Lee, J.H. Resveratrol Cross-Linked Chitosan Loaded with Phospholipid for Controlled Release and Antioxidant Activity. Int. J. Biol. Macromol. 2016, 93, 757–766. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved Chemical Stability and Cellular Antioxidant Activity of Resveratrol in Zein Nanoparticle with Bovine Serum Albumin-Caffeic Acid Conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef]
- Vo, A.; Feng, X.; Smith, W.C.; Zhu, D.; Patel, M.; Kozak, D.; Wang, Y.; Zheng, J.; Ashraf, M.; Xu, X. Analyzing Ophthalmic Suspension Particle Size Distributions Using Laser Diffraction: Placebo Background Subtraction Method. Int. J. Pharm. 2021, 598, 120401. [Google Scholar] [CrossRef] [PubMed]
- Pınar, S.G.; Oktay, A.N.; Karaküçük, A.E.; Çelebi, N. Formulation Strategies of Nanosuspensions for Various Administration Routes. Pharmaceutics 2023, 15, 1520. [Google Scholar] [CrossRef] [PubMed]
- del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Gómez, A. Formulación y Caracterización de Nano-Emulsiones de Aceite de Parafina Tipo Agua-En-Aceite (W/O). Ph.D. Thesis, Centro de Investigación en Materiales Avanzados, Nuevo León, Mexico, 2014. [Google Scholar]
- Pouton, C.W.; Porter, C.J.H. Formulation of Lipid-Based Delivery Systems for Oral Administration: Materials, Methods and Strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Nasr, M. Transdermal Delivery of Betahistine Hydrochloride Using Microemulsions: Physical Characterization, Biophysical Assessment, Confocal Imaging and Permeation Studies. Colloids Surf. B Biointerfaces 2013, 110, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Kaur, G. Leucaena Leucocephala (Lam.) Galactomannan Nanoparticles: Optimization and Characterization for Ocular Delivery in Glaucoma Treatment. Int. J. Biol. Macromol. 2019, 139, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Toropainen, E.; Fraser-Miller, S.J.; Novakovic, D.; Del Amo, E.M.; Vellonen, K.-S.; Ruponen, M.; Viitala, T.; Korhonen, O.; Auriola, S.; Hellinen, L.; et al. Biopharmaceutics of Topical Ophthalmic Suspensions: Importance of Viscosity and Particle Size in Ocular Absorption of Indomethacin. Pharmaceutics 2021, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993; Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2009.
- Wang, Q.; Zhang, X.; Wang, K.; Zhu, L.; Qiu, B.; Chen, X.; Lin, X.; Nie, Y. An In Vitro Model of Diabetic Retinal Vascular Endothelial Dysfunction and Neuroretinal Degeneration. J. Diabetes Res. 2021, 2021, 9765119. [Google Scholar] [CrossRef]
- Rokicki, D.; Zdanowski, R.; Lewicki, S.; Leśniak, M.; Suska, M.; Wojdat, E.; Skopińska-Różewska, E.; Skopiński, P. Experimental Immunology Inhibition of Proliferation, Migration and Invasiveness of Endothelial Murine Cells Culture Induced by Resveratrol. Cent. Eur. J. Immunol. 2014, 39, 449–454. [Google Scholar] [CrossRef]
- Losso, J.N.; Truax, R.E.; Richard, G. Trans-Resveratrol Inhibits Hyperglycemia-Induced Inflammation and Connexin Downregulation in Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2010, 58, 8246–8252. [Google Scholar] [CrossRef]
- Miranda, M.; Muriach, M.; Johnsen, S.; Bosch-Morell, F.; Araiz, J.; Romá, J.; Romero, F.J. Estrés Oxidativo En Un Modelo de Retinopatía Diabética Experimental: Tratamiento Con Antioxidantes. Arch. Soc. Esp. Oftalmol. 2004, 79, 289–294. [Google Scholar] [CrossRef]
- Soufi, F.G.; Mohammad-nejad, D.; Ahmadieh, H. Resveratrol Improves Diabetic Retinopathy Possibly through Oxidative Stress—Nuclear Factor κB—Apoptosis Pathway. Pharmacol. Rep. 2012, 64, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Diaz Arce, D. Hiperglicemia y Estrés Oxidativo En El Paciente Diabético. Rev. Cuba. Investig. Bioméd. 2006, 25. [Google Scholar]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol Exhibits an Effect on Attenuating Retina Inflammatory Condition and Damage of Diabetic Retinopathy via PON1. Exp. Eye Res. 2019, 181, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Anti-diabetic Effects of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef]
- Méndez-del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Lizárraga-Valdez, R. Effect of Resveratrol Administration on Metabolic Syndrome, Insulin Sensitivity, and Insulin Secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Perez, J.; Lopera-Echavarría, A.M.; Arevalo-Alquichire, S.; Araque-Marín, P.; Londoño, M.E. Development of a Resveratrol Nanoformulation for the Treatment of Diabetic Retinopathy. Materials 2024, 17, 1420. https://doi.org/10.3390/ma17061420
Gonzalez-Perez J, Lopera-Echavarría AM, Arevalo-Alquichire S, Araque-Marín P, Londoño ME. Development of a Resveratrol Nanoformulation for the Treatment of Diabetic Retinopathy. Materials. 2024; 17(6):1420. https://doi.org/10.3390/ma17061420
Chicago/Turabian StyleGonzalez-Perez, Juliana, A. M. Lopera-Echavarría, Said Arevalo-Alquichire, Pedronel Araque-Marín, and Martha E. Londoño. 2024. "Development of a Resveratrol Nanoformulation for the Treatment of Diabetic Retinopathy" Materials 17, no. 6: 1420. https://doi.org/10.3390/ma17061420
APA StyleGonzalez-Perez, J., Lopera-Echavarría, A. M., Arevalo-Alquichire, S., Araque-Marín, P., & Londoño, M. E. (2024). Development of a Resveratrol Nanoformulation for the Treatment of Diabetic Retinopathy. Materials, 17(6), 1420. https://doi.org/10.3390/ma17061420