Cell Guidance by 3D-Gradients in Hydrogel Matrices: Importance for Biomedical Applications
Abstract
:1. Introduction
1.1. Native Extracellular Matrix
1.1.1. Cells interacting with the ECM
1.1.2. Gradients for cell guidance in the native ECM
1.1.3. Cellular responses to gradients
1.2. Gradients for Biomedical or Technical Applications

1.3. Hydrogel Matrices as Replacements for Natural Extracellular Matrices
1.3.1. Gradients in 3D-hydrogel matrices
Soluble gradients within 3D-hydrogel matrices
2D gradients on 3D-hydrogel matrices
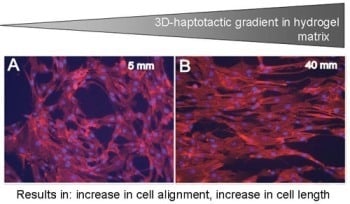
Covalent 3D-haptotactic gradients within hydrogel matrices

1.4. Summary
| Gradient type/gradient production | Applications of gradients | Source | |
|---|---|---|---|
| Soluble gradients within 3D-hydrogels | - 3D-Gradients of soluble molecules (e.g. growth factors, cytokines or hormones) within a hydrogel matrix. Gradient establishment is mostly based on diffusion. - microfluidics or macromolecular fluid devices - diffusion from chambers separated by semi-permeable membranes - microsphere-based delivery | - Gradient systems used to investigate neurite extension, angiogenesis, homing of lymphocytes- Cells cultured on top or within the hydrogel matrices - Assessment of cell alignment, migration, differentiation of stem cells - Guidance along the fibrous structures of the hydrogel matrices | [51], [77], [78], [124,125,126,127,128] |
| 2D-Surface gradients on top of 3D-hydrogels | Matrix-bound (= haptotactic) gradients of biologically active molecules | - Direction of cell growth and migration to improve integration of biomedical implants and therefore facilitate healing responses - Cells cultured on top - Assessment of cell alignment, migration and differentiation | [129], [130] |
| Haptotactic 3D-gradients within hydrogels | Matrix-immobilized 3D-gradients of adhesion or extracellular matrix molecules or growth factors within a hydrogel matrix. Gradient generation: - diffusion-based or gradient mixing devices both followed by photo- or chemical immobilization | - Gradients are used to investigate 3D-cell behaviour: invasion, vascular tube formation, neurite extension Cells cultured on top or within the hydrogel matrices - Assessment of cell alignment, migration and guidance along the fibers of gradient hydrogel matrices | [8], [62], [93], [131,132,133,134] |
| 3D-haptotactic combined with 3D-soluble gradients in hydrogels | Matrix-immobilized 3D-gradients of adhesion or extracellular matrix molecules or growth factors within a hydrogel matrix. Gradient generation: - diffusion-based or gradient mixing devices both followed by photo- or chemical immobilization | - Gradients are used to investigate 3D-cell behaviour: invasion, vascular tube formation, neurite extension Cells cultured on top or within the hydrogel matrices - Assessment of cell alignment, migration and guidance along the fibers of gradient hydrogel matrices | [8], [62], [93], [131,132,133,134] |
2. Discussion and Outlook: Applications in Biomedicine

- (i)
- Wound healing is one of the major clinical issues with increasing age of the population. Often underlying diseases such as diabetes or cardiovascular dysfunctions combined with medications induce chronic wounds that need sophisticated care and treatment [140]. Here bulk materials usable as wound filler or in combination with wound dressings providing gradients of soluble and/or matrix-bound guidance cues would be highly desirable (Figure 3A). Soluble gradients of cytokines would attract blood-derived macrophages for wound cleaning and stem cells that improve the healing response. Matrix-bound guidance cues on slow degrading fibrous matrices could overcome the inherent shortage of appropriate matrix molecules within a chronic wound as the endogenous matrix is degraded by an excess of wound-secreted proteolytic enzymes.
- (ii)
- Another application would be to direct endothelial cells within vascular grafts or neural cells in nerve guide tubes by one-sided matrix-bound gradients of growth factors and favorable adhesion sites (Figure 3B). The motivation is that many studies have shown that endothelialization of implanted vascular grafts in humans occurs very slow and often incomplete such that the graft materials remain blood-exposed [141,142]. These materials are often thrombogenic and induce re-stenosis in about 30 % percentage of patients [143]. Gradient-type guidance of endothelial cells through the length and/or through the wall pores of the vascular graft might increase and shorten the time required for vascularization. Often traumatic experiences are accompanied with injuries in the peripheral nervous system that need to be treated in order to increase the probability of regain of function. In small injuries (< 4 mm) the injured nerve can be reconnected by direct end-to-end suturing however when larger nerve pieces are missing the remaining ends are sutured within nerve guide tubes or nerve conduits [144,145]. These polymer tubing provide guidance and protection for newly sprouting proximal nerve ends and allow reconnection with the proper target organ. In order to increase the speed and the quality of nerve regeneration it would be highly desirable to extend the studies performed by [8] that demonstrated the need of synergistic guidance cues provided by laminin-1 in combination with growth factor(s) NGF in a one-sided gradient fashion along the guidance structures.
- (iii)
- In certain cases also two-sided gradients of guidance cues in a bulk material might be an interesting option. Cortical bone defects heal when the injury, trauma or bone loss lies within the limits of bone regeneration. Bone defects above a critical-size do not heal with bone formation instead bone is replaced by scar tissue which can not provide the load-bearing functions of bone [146]. In order to stimulate bone formation even in critical-size bone defects bone substitute materials such as hydroxyapatite, tricalcium phosphate foams, bioglasses, composite metals [146,147] and many others are filled with bone morphogenetic protein (BMP)-2. As BMP-2 is a very potent inducer of bone formation dosage and correct placing are critical issues, therefore it might be an interesting thought to introduce BMP-2 or plasmids that lead to production of BMP-2 after transfection of wound cells in a two-sided gradient manner in order to attract bone forming cells towards the center of the bone substitute material.
3. Conclusions
Acknowledgements
References and Notes
- Detrich, H.W., 3rd. Fluorescent proteins in zebrafish cell and developmental biology. Methods Cell Biol. 2008, 85, 219–241. [Google Scholar] [PubMed]
- Ochoa, O.; Torres, F.M.; Shireman, P.K. Chemokines and diabetic wound healing. Vascular 2007, 15, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Xu, X.; Hereld, D. Chemotaxis, chemokine receptors and human disease. Cytokine 2008, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, S.; Perris, R. Proteoglycan control of cell movement during wound healing and cancer spreading. Matrix Biol. 2005, 24, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Lundkvist, A.; Lee, S.; Iruela-Arispe, L.; Betsholtz, C.; Gerhardt, H. Growth factor gradients in vascular patterning. Novartis Found Symp. 2007, 283, 194–201; discussion 201–206, 238–241. [Google Scholar] [PubMed]
- Friedl, P.; Brocker, E.B. T Cell migration in three-dimensional extracellular matrix: Guidance by polarity and sensations. Dev Immunol 2000, 7, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.G. Neural map specification by gradients. Curr. Opin. Neurobiol. 2006, 16, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Dodla, M.D.; Bellamkonda, R.V. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials 2008, 29, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Sanford, S.D.; Gatlin, J.C.; Hoekfelt, T.; Pfenninger, K.H. Growth cone responses to growth and chemotropic factors. Europ. J. Neurosci. 2008, 28, 268–278. [Google Scholar] [CrossRef]
- Kima, M.S.; Khangb, G.; Lee, H.B. Gradient polymer surfaces for biomedical applications. Prog. Polym. Sci. 2008, 33, 138–164. [Google Scholar] [CrossRef]
- Hsu, S.; Thakar, R.; Li, S. Haptotaxis of endothelial cell migration under flow. Methods Mol. Med. 2007, 139, 237–250. [Google Scholar] [PubMed]
- Waite, J.H.; Lichtenegger, H.C.; Stucky, G.D.; Hansma, P. Exploring molecular and mechanical gradients in structural bioscaffolds. Biochemistry 2004, 43, 7653–7662. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, S.M.; Lee, S.; Spencer, N.D. Submicrometer structure of surface-chemical gradients prepared by a two-step immersion method. Langmuir 2006, 22, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, T.B.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Genzer, J.; Bhat, R.R. Surface-bound soft matter gradients. Langmuir 2008, 24, 2294–2317. [Google Scholar] [CrossRef] [PubMed]
- Riepl, M.; Ostblom, M.; Lundstrom, I.; Svensson, S.C.; van der Gon, A.W.D.; Schaferling, M.; Liedberg, B. Molecular gradients: An efficient approach for optimizing the surface properties of biomaterials and biochips. Langmuir 2005, 21, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J.; Tam, P.P. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 2009, 136, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Palmeirim, I.; Rodrigues, S.; Dale, J.K.; Maroto, M. Development on time. Adv. Exp. Med. Biol. 2008, 641, 62–71. [Google Scholar] [PubMed]
- Lewis, R.A.; Gagnon, J.A.; Mowry, K.L. PTB/hnRNP I is required for RNP remodeling during RNA localization in Xenopus oocytes. Mol. Cell Biol. 2008, 28, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Fu, L.; Fiorentino, M.; Matsuda, H.; Das, B.; Shi, Y.B. Differential regulation of cell type-specific apoptosis by stromelysin-3: A potential mechanism via the cleavage of the laminin receptor during tail resorption in xenopus laevis. J. Biol. Chem. 2009, 284, 18545–18556. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.I.; Mouw, J.K.; Weaver, V.M. Biomechanical regulation of cell orientation and fate. Oncogene 2008, 27, 6981–6993. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Aspects Med. 2008, 29, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Schoenherr, E.; Hausser, H.J. Extracellular matrix and cytokines: A functional unit. Dev. Immunol. 2000, 7, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Ubersax, L.; Merkle, H.P.; Meinel, L. Biopolymer based growth factor delivery for tissue repair: From natural concepts to engineered systems. Tissue Eng. Part B Rev. 2009. [Google Scholar] [CrossRef]
- Miner, J.H.; Li, C.; Patton, B.L. Laminins alpha2 and alpha4 in pancreatic acinar basement membranes are required for basal receptor localization. J. Histochem. Cytochem. 2004, 52, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Ingber, D.E. Micromechanical control of cell and tissue development: Implications for tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Choquet, D.; Felsenfeld, D.P.; Sheetz, M.P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 1997, 88, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Sero, J.E.; Mammoto, T.; Ingber, D.E. Methods for studying mechanical control of angiogenesis by the cytoskeleton and extracellular matrix. Methods Enzymol 2008, 443, 227–259. [Google Scholar]
- Mammoto, A.; Connor, K.M.; Mammoto, T.; Yung, C.W.; Huh, D.; Aderman, C.M.; Mostoslavsky, G.; Smith, L.E.H.; Ingber, D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 2009, 457, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Pankov, R.; Yamada, K.M. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 2002, 14, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tytell, J.G.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Banno, A.; Ginsberg, M.H. Integrin activation. Biochem. Soc. Trans. 2008, 36, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Legate, K.R.; Zent, R.; Fässler, R. The tail of integrins, talin, and kindlins. Science 2009, 324, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 2009, 10, 21–33. [Google Scholar] [CrossRef]
- Arnaout, M.A.; Goodman, S.L.; Xiong, J.P. Structure and mechanics of integrin-based cell adhesion. Curr. Opin. Cell Biol. 2007, 19, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Even-Ram, S. Integrin regulation of growth factor receptors. Nat. Cell Biol. 2002, 4, E75–E76. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.H.; Springer, T.A. Integrin structures and conformational signaling. Curr. Opin. Cell Biol. 2006, 18, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol 2007, 8, 215:1–215:9. [Google Scholar] [CrossRef]
- Hynes, R.O. A reevaluation of integrins as regulators of angiogenesis. Nat. Med. 2002, 8, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 2008, 4, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Betsholtz, C. How do endothelial cells orientate? Exs 2005, 94, 3–15. [Google Scholar] [PubMed]
- Mortimer, D.; Fothergill, T.; Pujic, Z.; Richards, L.J.; Goodhill, G.J. Growth cone chemotaxis. Trends Neurosci. 2008, 31, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Mechanisms of cell migration in the vertebrate embryo. Cell Differ. 1984, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Zanker, K.S.; Brocker, E.B. Cell migration strategies in 3-D extracellular matrix: Differences in morphology, cell matrix interactions, and integrin function. Microsc. Res. Tech. 1998, 43, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Khademhosseini, A.; Langer, R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir 2004, 20, 5153–5156. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Elkin, J.T.; Reichert, W.M. Directed cell migration on fibronectin gradients: Effect of gradient slope. Exp. Cell Res. 2006, 312, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Kim, D.H.; Reichert, W.M. Haptotactic gradients for directed cell migration: Stimulation and inhibition using soluble factors. Comb. Chem. High. Throughput Screen 2009, 12, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, A.S.; Jabbari, E. Analysis of cell locomotion on ligand gradient substrates. Biotechnol. Bioeng. 2009, 103, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Firtel, R.A.; Chung, C.Y. The molecular genetics of chemotaxis: Sensing and responding to chemoattractant gradients. Bioessays 2000, 22, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Thakar, R.; Liepmann, D.; Li, S. Effects of shear stress on endothelial cell haptotaxis on micropatterned surfaces. Biochem. Biophys. Res. Commun. 2005, 337, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cui, Z.; Urban, J.P.G. Nutrient gradients in engineered cartilage: Metabolic kinetics measurement and mass transfer modeling. Biotechnol. Bioeng. 2008, 101, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Dafni, H.; Landsman, L.; Schechter, B.; Kohen, F.; Neeman, M. MRI and fluorescence microscopy of the acute vascular response to VEGF165: Vasodilation, hyper-permeability and lymphatic uptake, followed by rapid inactivation of the growth factor. NMR Biomed. 2002, 15, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Gurdon, J.B.; Bourillot, P.Y. Morphogen gradient interpretation. Nature 2001, 413, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Fleury, M.E. Interstitial flow and its effects in soft tissues. Annu. Rev. Biomed. Eng. 2007, 9, 229–256. [Google Scholar] [CrossRef] [PubMed]
- Semino, C.E.; Kamm, R.D.; Lauffenburger, D.A. Autocrine EGF receptor activation mediates endothelial cell migration and vascular morphogenesis induced by VEGF under interstitial flow. Exp. Cell Res. 2006, 310, 289–298. [Google Scholar]
- Helm, C.L.; Fleury, M.E.; Zisch, A.H.; Boschetti, F.; Swartz, M.A. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc. Natl. Acad. Sci. USA 2005, 102, 15779–15784. [Google Scholar] [CrossRef] [PubMed]
- Rosoff, W.J.; Urbach, J.S.; Esrick, M.A.; McAllister, R.G.; Richards, L.J.; Goodhill, G.J. A new chemotaxis assay shows the extreme sensitivity of axons to molecular gradients. Nat. Neurosci. 2004, 7, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.C.; Quinn, T.M. Solute diffusivity correlates with mechanical properties and matrix density of compressed articular cartilage. Arch. Biochem. Biophys. 2005, 442, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, T.; Hanseler, P.; Grant, B.; Hall, H. The induction of cell alignment by covalently immobilized gradients of the 6th Ig-like domain of cell adhesion molecule L1 in 3D-fibrin matrices. Biomaterials 2009, 27, 4503–4512. [Google Scholar]
- Fleury, M.E.; Boardman, K.C.; Swartz, M.A. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys. J. 2006, 91, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ruhrberg, C.; Gerhardt, H.; Golding, M.; Watson, R.; Ioannidou, S.; Fujisawa, H.; Betsholtz, C.; Shima, D.T. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002, 16, 2684–2698. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B. The specificity of interactions between proteins and sulfated polysaccharides. An Acad. Bras. Cienc. 2005, 77, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Bellamkonda, R.V. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials 2006, 27, 3515–3518. [Google Scholar] [PubMed]
- Goerges, A.L.; Nugent, M.A. pH regulates vascular endothelial growth factor binding to fibronectin. J. Biol. Chem. 2004, 279, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Goerges, A.L.; Nugent, M.A. pH regulates vascular endothelial growth factor binding to fibronectin: A mechanism for control of extracellular matrix storage and release. J. Biol. Chem. 2004, 279, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Guimond, S.; Maccarana, M.; Olwin, B.B.; Lindahl, U.; Rapraeger, A.C. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J. Biol. Chem. 1993, 268, 23906–23914. [Google Scholar] [PubMed]
- Friesel, R.E.; Maciag, T. Molecular mechanisms of angiogenesis: Fibroblast growth factor signal transduction. FASEB J. 1995, 9, 919–925. [Google Scholar] [PubMed]
- Klint, P.; Claesson-Welsh, L. Signal transduction by fibroblast growth factor receptors. Front. Biosci. 1999, 4, D165–D177. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.J.; Stringer, S.E. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 2001, 114 (Pt 5), 853–865. [Google Scholar] [PubMed]
- Ruhrberg, C. Growing and shaping the vascular tree: multiple roles for VEGF. Bioessays 2003, 25, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Stephens, L.; Milne, L.; Hawkins, P. Moving towards a better understanding of chemotaxis. Curr. Biol. 2008, 18, R485–R494. [Google Scholar] [CrossRef] [PubMed]
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962, 115, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Zicha, D.; Dunn, G.A.; Brown, A.F. A new direct-viewing chemotaxis chamber. J. Cell Sci. 1991, 99 (Pt 4), 769–775. [Google Scholar] [PubMed]
- Li, G.N.; Liu, J.; Hoffman-Kim, D. Multi-molecular gradients of permissive and inhibitory cues direct neurite outgrowth. Ann. Biomed. Engin. 2008, 36, 889–904. [Google Scholar] [CrossRef]
- Taylor, S.J.; Sakiyama-Elbert, S.E. Effect of controlled delivery of neurotrophin-3 from fibrin on spinal cord injury in a long term model. J. Control Release 2006, 116, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Harley, B.A.C.; Kim, H.D.; Zaman, M.H.; Yannas, I.V.; Lauffenburger, D.A.; Gibson, L.J. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys. J. 2008, 95, 4013–4024. [Google Scholar] [CrossRef] [PubMed]
- Parent, C.A.; Blacklock, B.J.; Froehlich, W.M.; Murphy, D.B.; Devreotes, P.N. G protein signaling events are activated at the leading edge of chemotactic cells. Cell 1998, 95, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.G.; Kolega, J. Endothelial cell protrusion and migration in three-dimensional collagen matrices. Cell Motil. Cytoskeleton 2006, 63, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Harms, B.D.; Bassi, G.M.; Horwitz, A.R.; Lauffenburger, D.A. Directional persistence of EGF-induced cell migration is associated with stabilization of lamellipodial protrusions. Biophys. J. 2005, 88, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-D.; Guo, T.W.; Wu, A.P.; Wells, A.; Gertler, F.B.; Lauffenburger, D.A. Epidermal growth factor–induced enhancement of glioblastoma cell migration in 3D arises from an intrinsic increase in speed but an extrinsic matrixand proteolysis-dependent increase in persistence. Mol. Biol. Cell 2008, 19, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Q.; Cohan, C.S. How actin filaments and microtubules steer growth cones to their targets. J. Neurobiol. 2004, 58, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Chilton, J.K. The specific targeting of guidance receptors within neurons: Who directs the directors? Dev. Biol. 2009, 327, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.M.; Mosto, K.E. From cells to organs: Building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008, 9, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gundersen, G.G. Beyond polymer polarity: How the cytoskeleton builds a polarized cell. Nat. Rev. 2008, 9, 860–873. [Google Scholar] [CrossRef]
- Zaman, M.H.; Kamm, R.D.; Matsudaira, Y.P.; Lauffenburger, D.A. Computational model for cell migration in three-dimensional matrices. Biophys. J. 2005, 89, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Mallet, D.G.; Pettet, G.J. A mathematical model of integrin-mediated haptotactic cell migration. Bull. Math. Biol. 2006, 68, 231–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hypolite, C.L.; McLernon, T.L.; Adams, D.N.; Chapman, K.E.; Herbert, C.B.; Huang, C.C.; Distefano, M.D.; Hu, W.S. Formation of microscale gradients of protein using heterobifunctional photolinkers. Bioconjug. Chem. 1997, 8, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Kipper, M.J.; Kleinman, H.K.; Wang, F.W. Covalent surface chemistry gradients for presenting bioactive peptides. Anal. Biochem. 2007, 363, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ratner, B.D.; Sage, E.H.; Jiang, S. Endothelial cell migration on surface-density gradients of fibronectin, VEGF, or both proteins. Langmuir 2007, 23, 11168–11173. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wan, L.; Wu, J.; Gao, C. Microscale control over collagen gradient on poly(l-lactide) membrane surface for manipulating chondrocyte distribution. Colloids Surf. B: Biointerfaces 2008, 67, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Yua, L.M.Y.; Wosnick, J.H.; Shoichet, M.L. Miniaturized system of neurotrophin patterning for guided regeneration. J. Neurosci. Methods 2008, 171, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Iida, Y.; Otani, Y.; Hirano, Y.; Tabata, Y. Adhesion behavior of human adipo-stromal cells on self-assembled monolayers with different surface densities or gradients of RGD peptide. J. Biomaterials Sci. 2009, 20, 495–510. [Google Scholar] [CrossRef]
- Kang, C.E.; Gemeinhart, E.J.; Gemeinhart, R.A. Cellular alignment by grafted adhesion peptide surface density gradients. J. Biomed. Mater. Res. A 2004, 71, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.T.; Jeon, N.L.; Huang, S.; Kane, R.S.; Wargo, C.J.; Choi, I.S.; Ingber, D.E.; Whitesides, G.M. Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proc. Natl. Acad. Sci. USA 2000, 97, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.M.; Folch, A. Biomolecular gradients in cell culture systems. Lab Chip 2008, 8, 34–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Baac, H.; Song, S.H.; Lee, S.D.; Park, D.; Kim, S.J. The topographical guidance of neurons cultured on holographic photo-responsive polymer. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004, 7, 4970–4973. [Google Scholar] [PubMed]
- Guido, S.; Tranquillo, R.T. A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. Correlation of fibroblast orientation and gel birefringence. J. Cell Sci. 1993, 105 (Pt 2), 317–331. [Google Scholar] [PubMed]
- Scholl, M.; Sprossler, C.; Denyer, M.; Krause, M.; Nakajima, K.; Maelicke, A.; Knoll, W.; Offenhausser, A. Ordered networks of rat hippocampal neurons attached to silicon oxide surfaces. J. Neurosci. Methods 2000, 104, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Loesberg, W.A.; te Riet, J.; van Delft, F.C.; Schon, P.; Figdor, C.G.; Speller, S.; van Loon, J.J.; Walboomers, X.F.; Jansen, J.A. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials 2007, 28, 3944–3951. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Kiick, K.L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Anseth, K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Zisch, A.H.; Lutolf, M.P.; Hubbell, J.A. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc. Pathol. 2003, 12, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Lutholf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.C.; Dalton, P.D.; Shoichet, M.S.; Tator, C.H. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials 2006, 27, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Weaver, V.M. The tension mounts: mechanics meets morphogenesis and malignancy. J. Mammary Gland Biol. Neoplasia 2004, 9, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Semler, E.J.; Lancin, P.A.; Dasgupta, A.; Moghe, P.V. Engineering hepatocellular morphogenesis and function via ligand-presenting hydrogels with graded mechanical compliance. Biotechnol. Bioeng. 2005, 89, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Hirt, T.D.; Neuenschwander, P.; Suter, U.W. Synthesis of degradable, biocompatible, and tough blockcopol yesterurethanes. Matromol. Chem. Phys. 1996, 197, 4253–4268. [Google Scholar] [CrossRef]
- Yang, Y.; Kaufman, L.J. Rheology and confocal reflectance microscopy as probes of mechanical properties and structure during collagen and collagen/hyaluronan self-assembly. Biophys. J. 2009, 96, 1566–1585. [Google Scholar] [CrossRef] [PubMed]
- Raeber, G.P.; Lutolf, M.P.; Hubbell, J.A. Molecularly engineered PEG hydrogels: A novel model system for proteolytically mediated cell migration. Biophys. J. 2005, 89, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Urech, L.; Bittermann, A.G.; Hubbell, J.A.; Hall, H. Mechanical properties, proteolytic degradability and biological modifications affect angiogenic process extension into native and modified fibrin matrices in vitro. Biomaterials 2005, 26, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Pike, D.B.; Cai, S.; Pomraning, K.R.; Firpo, M.A.; Fisher, R.J.; Shu, X.Z.; Prestwich, G.D.; Peattie, R.A. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronic hydrogels containing VEGF and bFGF. Biomaterials 2006, 27, 5242–5251. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Ehrbar, M.; Metters, A.; Zammaretti, P.; Hubbell, J.A.; Zisch, A.H. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J. Control Release 2005, 101, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Kobayashi, H.; Tabata, Y. Enhanced angiogenesis through controlled release of basic fibroblast growth factor from peptide amphiphile for tissue regeneration. Biomaterials 2006, 27, 5836–5844. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Masanori, F.; Kiyohaya, O.; Hattori, H.; Kikuchi, M.; Maehara, T. Controlled release of FGF-2 and paclitaxol from chitosan hdrogels and their subsequent effects on wound repair, angiogenesis, and tumor growth. Curr. Drug. Delivery 2006, 3, 351–358. [Google Scholar] [CrossRef]
- Ruszczak, R.; Friess, W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv. Drug Delivery Rev. 2003, 55, 1679–1689. [Google Scholar] [CrossRef]
- Wallace, D.G.; Rosenblatt, J. Collagen gel systems for sustained delivery and tissue engineering. Adv. Drug Delivery Rev. 2003, 55, 1631–1649. [Google Scholar] [CrossRef]
- Abhyankar, V.V.; Lokuta, M.A.; Huttenlocher, A.; Beebe, D.J. Characterization of a membrane-based gradient generator for use in cell-signaling studies. Lab Chip 2006, 6, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Barkefors, I.; Thorslund, S.; Nikolajeff, F.; Kreuger, J. A fluidic device to study directional angiogenesis in complex tissue and organ culture models. Lab Chip 2009, 9, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.F. Endothelial mechanisms of flow-mediated athero-protection and susceptibility. Circ. Res. 2008, 101. [Google Scholar]
- Knapp, D.M.; Helou, E.F.; Tranquillo, R.T. A fibrin or collagen gel assay for tissue cell chemotaxis: assessment of fibroblast chemotaxis to GRGDSP. Exp. Cell Res. 1999, 247, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wenk, E.; Zhang, X.; Meinel, L.; Vunjak-Novakovic, G.; Kaplan, D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control. Release 2009, 134, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ilkhanizadeh, S.; Teixeira, A.I.; Hermanson, O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 2007, 28, 3936–3943. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.G.; Miller, E.D.; Fisher, G.W.; Walker, L.M.; Weiss, L.E. Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell organization. Biomaterials 2005, 26, 6762–6770. [Google Scholar] [CrossRef] [PubMed]
- Polizzotti, B.D.; Fairbanks, B.D.; Anseth, K.S. Three-dimensional biochemical patterning of click-based composite hydrogels via thiolene photopolymerization. Biomacromolecules 2008, 9, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- DeLong, S.A.; Gobin, A.S.; West, J.L. Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J. Control. Release 2005, 109, 139–148. [Google Scholar] [CrossRef] [PubMed]
- DeLong, S.A.; Moon, J.J.; West, J.L. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 2005, 26, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Dodla, M.C.; Bellamkonda, R.V. Anisotropic scaffolds facilitate enhanced neurite extension in vitro. J. Biomed. Mater. Res. A 2006, 78A, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.M.; Becker, J.C.; Siu, C.H.; Lemmon, V.P.; Cheresh, D.A.; Pancook, J.D.; Zhao, X.; Reisfeld, R.A. Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin alpha v beta 3. J. Cell Biol. 1996, 132, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Felding-Habermann, B.; Silletti, S.; Mei, F.; Siu, C.H.; Yip, P.M.; Brooks, P.C.; Cheresh, D.A.; O'Toole, T.E.; Ginsberg, M.H.; Montgomery, A.M. A single immunoglobulin-like domain of the human neural cell adhesion molecule L1 supports adhesion by multiple vascular and platelet integrins. J. Cell Biol. 1997, 139, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- Blaess, S.; Kammerer, R.A.; Hall, H. Structural analysis of the sixth immunoglobulin-like domain of mouse neural cell adhesion molecule L1 and its interactions with alpha(v)beta3, alpha(IIb)beta3, and alpha5beta1 integrins. J. Neurochem. 1998, 71, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Schense, J.C.; Hubbell, J.A. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug. Chem. 1999, 10, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Hubbell, J.A. Matrix-bound sixth Ig-like domain of cell adhesion molecule L1 acts as an angiogenic factor by ligating alphavbeta3-integrin and activating VEGF-R2. Microvasc. Res. 2004, 68, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Rimann, M.H. Non-viral and local gene medicine for improvement of cutanous wound healing. Gene Ther. Mol. Biol. 2009, 13, 53–63. [Google Scholar]
- Zilla, P.; Deutsch, M.; Meinhart, J.; Puschmann, R.; Eberl, T.; Minar, E.; Dudczak, R.; Lugmaier, H.; Schmidt, P.; Noszian, I.; et al. Clinical in vitro endothelialization of femoropopliteal bypass grafts: An actuarial follow-up over three years. J. Vasc. Surg. 1994, 19, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; von Oppell, U.; Deutsch, M. The endothelium: A key to the future. J. Card. Surg. 1993, 8, 32–60. [Google Scholar] [CrossRef] [PubMed]
- Radke, P.W.; Kaiser, A.; Frost, C.; Sigwart, U. Outcome after treatment of coronary in-stent restenosis; Results from a systematic review using meta-analysis techniques. Eur. Heart J. 2003, 24, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Dvali, L.T.; Myckatyn, T.M. End-to-side nerve repair: Review of the literature and clinical indications. Hand Clin. 2008, 24, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Schlosshauer, B.; Dreesmann, L.; Schaller, H.E.; Sinis, N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery 2006, 59, 740–747; discussion 747–748. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ries, J.; Gelse, K.; Kloss, F.; von der Mark, K.; Wiltfang, J.; Neukam, F.W.; Schneider, H. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: A comparison of adenoviral vectors and liposomes. Gene Ther. 2003, 10, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Smolec, O.; Krpan, D.; Babic, D.; Vnuk, D.; Kreszinger, M.; Pirkic, B.; Hock, K. Healing of critical size defect on diaphiseal bone in rabbit by using free omental graft. Bone 2008, 43, S78. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lühmann, T.; Hall, H. Cell Guidance by 3D-Gradients in Hydrogel Matrices: Importance for Biomedical Applications. Materials 2009, 2, 1058-1083. https://doi.org/10.3390/ma2031058
Lühmann T, Hall H. Cell Guidance by 3D-Gradients in Hydrogel Matrices: Importance for Biomedical Applications. Materials. 2009; 2(3):1058-1083. https://doi.org/10.3390/ma2031058
Chicago/Turabian StyleLühmann, Tessa, and Heike Hall. 2009. "Cell Guidance by 3D-Gradients in Hydrogel Matrices: Importance for Biomedical Applications" Materials 2, no. 3: 1058-1083. https://doi.org/10.3390/ma2031058
APA StyleLühmann, T., & Hall, H. (2009). Cell Guidance by 3D-Gradients in Hydrogel Matrices: Importance for Biomedical Applications. Materials, 2(3), 1058-1083. https://doi.org/10.3390/ma2031058