Our primary interest in dendrimers is their use as proteomimetics, and specifically as substitutes for lens crystallin proteins [
33,
34]. While many studies on PAMAM dendrimers often focus on ionic (i.e. amine or carboxyl terminated) dendrimers because of their potential uses in controlled drug or gene delivery, we selected G
5OH (hydroxyl-terminated) PAMAM dendrimers to study the effect of hydrophobicity on their biological behaviors as they are more suited to our intended ophthalmic applications. In general, dendrimer generation number accounts for the size of the dendrimer while surface charge and functionality are two major factors which influence the properties of dendrimers. If the effect of charge is negated, the influence of surface functionality, that is, hydrophobic effects in our case, would dominate the responses of the dendrimers.
2.1. Dendrimer Modification and Characterization
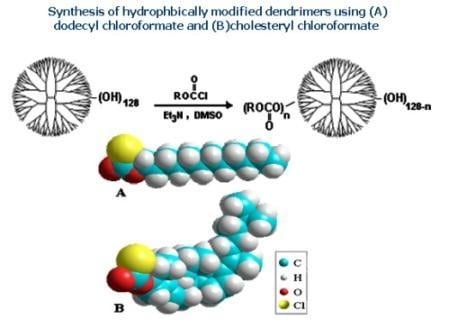
In this study, a series of hydrophobic modifications were carried out by conjugating the G
5OH dendrimer with either dodecyl chloroformate (a flexible aliphatic chain) or cholesteryl chloroformate (a rigid lipid found in abundance in biological systems). The amount of dodecyl chloroformate was adjusted to 5, 10 and 20 mole % of dendrimer hydroxyl groups, and the amount of cholesteryl chloroformate was 0.86, 2, 5, and 20 mole % of dendrimer hydroxyl groups. These molar ratios were based on the assumption that the G
5OH dendrimer contained 128 end-terminal OH groups. Commercially available PAMAM dendrimers are known to have structural defects, reducing the number of available end groups and molecular weight. GPC analysis of the unmodified dendrimer (supporting information) gave a molecular weight of 28.5 kD and a polydispersity of 1.007, indicating that the material was of high quality. The corresponding modified dendrimers were designated as D1, D2, D3 and C1, C2, C3, C4, respectively. As shown in
Table 1 and
Figure 1, structural analysis by
1H NMR data indicated that the conjugation of the G
5OH with dodecyl chloroformate followed a stoichiometric relationship in the investigated range. It was found that the maximum amount of cholesteryl moieties that could be introduced into the dendrimer was less than 5%. It is probable that steric hindrance caused by the bulky and rigid configuration of the cholesteryl group limited the amount of substitution. All the obtained products showed affinity to polar solvents such as dimethylsulfoxide (DMSO), dimethylformamide (DMF), and tetrahydrofuran (THF). It was found that high water solubility of the G
5OH dendrimer was no longer observed if more than 5% of hydroxyl groups were substituted with the dodecyl groups or 2% were substituted with the cholesteryl groups. In contrast to the unmodified G
5OH dendrimer that possesses a hydrodynamic diameter of 5.8 nm, the hydrophobized derivatives, through hydrophobic association, formed aggregates with hydrodynamic diameters ranging from 8 nm to 14 nm after sonication in physiological solutions. Their sizes and morphologies can be altered by many factors including the level of derivation, temperature, buffer, concentration, and sample preparation method. A detailed aggregation behavior of the water-soluble amphiphile C2 has been reported previously [
39]. In general, the aggregates formed by cholesteryl dendrimers were larger than those formed by the dodecyl dendrimers.
Table 1.
The physicochemical properties of hydrophobically modified and unmodified dendrimers.
Table 1.
The physicochemical properties of hydrophobically modified and unmodified dendrimers.
| Sample #(a) | % Hydrophobized groups(b) | Solubility in water (c) | Hydrodynamic diameter (d) (nm) |
|---|
| G5OH | 0 | S | 5.86 |
| D1 | 3.8 | S | 8.2 |
| D2 | 11.5 | IS | 9.6 |
| D3 | 20.5 | IS | 10.2 |
| C1 | 0.86 | S | 6.0 |
| C2 | 1.56 | S | 12.2 |
| C3 | 3.75 | IS | 13.8 |
| C4 | 3.2 | IS | -- |
Figure 1.
Partially hydrophobic modification of the G5OH dendrimer with dodecyl (Ο) or cholesteryl (Δ) chloroformate (the percentage of hydroxyl groups hydrophobized was determined by the NMR ratio of the resonance of dodecyl methyl proton at 0.83 ppm or cholesteryl = CH6 proton at 5.3 ppm to the corresponding dendrimer nucleus NH proton at 7.78 ppm).
Figure 1.
Partially hydrophobic modification of the G5OH dendrimer with dodecyl (Ο) or cholesteryl (Δ) chloroformate (the percentage of hydroxyl groups hydrophobized was determined by the NMR ratio of the resonance of dodecyl methyl proton at 0.83 ppm or cholesteryl = CH6 proton at 5.3 ppm to the corresponding dendrimer nucleus NH proton at 7.78 ppm).
Nourse
et al. [
40] carried out an extensive physicochemical characterization on the G
5OH dendrimer, and reported that it had a partial specific volume slightly greater than a typical globular protein. They also reported that the G
5OH dendrimer behaved as a discrete particle in aqueous solutions. This observation agrees with our results as indicated by the light scattering data in
Table 1 for the G
5OH unmodified dendrimer.
As dendrimers increase in generation number, there is a transition from an open dome-shaped structure at lower generations to a closed spheroid shape at higher generations [
41,
42,
43]. This transition takes place between generations 2 and 5, so that by generation 5 the molecular structure of the higher generation dendrimers has been described as a soft spongy interior with well defined interior hydrophobic cavities surrounded by a dense outer shell, which in the case of the G
5OH dendrimer is hydrophilic. This dense hydrophilic outer shell is largely responsible for shielding of the dendrimer interior and preventing intra-molecular interactions.
At 34% w/w concentration, the rheological properties (storage and loss moduli) of G
5OH dendrimers closely matched those of the total lens crystallin protein soluble fraction from porcine lenses [
37]. Similarly, they matched the values of refractive index (~1.4) and density (~1.08). However, one key difference was noted in the rheological behavior of the dendrimer and the natural lens crystallins. The crystallins showed shear thinning and non-Newtonian behavior, indicating complex molecular interactions [
36], while the G
5OH dendrimers exhibited strictly Newtonian behavior [
42,
44]. These crystallin interactions allow for the formation of short range lattices and are considered important in both the transparency and the accommodation or focusing properties of the lens in vision. One method of overcoming these rheological differences and creating dendrimer interaction is to conjugate hydrophobic surface molecules to the dendrimers. In addition to causing particle interaction and affecting rheological properties of the dendrimers, the hydrophobic derivatives help to trap the dendrimers in an amphiphilic gel, preventing them from escaping from a prosthetic lens refilling material, thus decreasing the likelihood of dendrimers reaching the cornea or retina and causing an inflammatory response
in-vivo.
The thermal transitions of the G
5OH dendrimer and its hydrophobic derivatives were studied by DSC. As seen in
Figure 2(a), the dodecyl dendrimers with various degree of dodecyl substitution had similar glass transition temperatures (T
g) as that of the G
5OH dendrimer (12.3 °C). A crystallization exotherm was observed at –15.1 °C in the DSC profiles of D3, suggesting that 20% dodecyl modified dendrimer showed crystallization to some extent. None of the cholesteryl modified dendrimers showed signs of crystallization as seen in
Figure 2(b). The
Tg of C1, C2 and C3 were 14.5, 20.3 and 16.5 °C, respectively, which were higher than that of the unmodified dendrimer. The curve of the sample C4 showed multiple transitions, implying that this sample was a mixture of the dendrimers with a wide range of different levels of cholesteryl modification. As the glass transition temperatures of all the samples tested were far below 37 °C, they behaved as liquids under the experimental condition for cell viability testing.
Figure 2.
DSC profiles of hydrophobically modified and unmodified dendrimers. (a) scans of the third cooling (top) and heating (bottom) for the G5OH dendrimer and the dodecyl dendrimers; (b) scans of the third cooling for cholesteryl dendrimers, processed as the first derivative.
Figure 2.
DSC profiles of hydrophobically modified and unmodified dendrimers. (a) scans of the third cooling (top) and heating (bottom) for the G5OH dendrimer and the dodecyl dendrimers; (b) scans of the third cooling for cholesteryl dendrimers, processed as the first derivative.
Figure 3 shows the tracings from the reverse phase HPLC using a 5% to 95% acetonitrile gradient. With increasing hydrophobic modification, the dendrimers should require an increased organic content to be washed from the columns that would be evidenced by a shift to the right in the tracings. This shift is clearly seen in the cholesteryl derivatives. With the dodecyl derivatives, there is a clear shift between the unmodified G
5OH dendrimer and D1, or the 5% dodecyl dendrimer. However, D2 and D3, the 10% and 20% dodecyl derivatives, appeared to come off the C18 column at a lower acetonitrile concentration than D1. This observation suggests that the dodecyl chains interacting with the column were less than those conjugated on the samples, as indicated by the NMR results, which clearly show the stoichiometric derivatization of the dodecyl groups. One possible explanation is that the dodecyl chains were partially shielded, thus preventing their interaction with the column. Monte Carlo simulations and studies of dendrimers in various solvent conditions showed that in poor solvents, back-folding of the peripheral groups can be expected [
45,
46]. It is possible that the flexible dodecyl hydrophobic chains could actually back-fold into the more hydrophobic interior of the dendrimer, or aggregation in part could be caused by internalization of adjacent dendrimer dodecyl chains. Encapsulation of hydrophobic guest molecules by dendrimers has been shown by many studies, which was covered in a recent review [
4]. G
5OH PAMAM dendrimers have been employed in a noncovalent drug inclusion complex study where it was demonstrated that methotrexate was internalized by dialysis in distilled water [
47], indicating that these particular dendrimers are capable of this type of inclusion. This concept will be revisited when the rheology of these dendrimers is discussed.
Figure 3.
HPLC UV tracings at 230 nm of control and modified dendrimers analyzed on reverse phase C18 columns to show hydrophobicity. (a) G5OH unmodified dendrimer (b) C1 (c) C2 (d) C3 (e) D1 (f) D2 (g) D3.
Figure 3.
HPLC UV tracings at 230 nm of control and modified dendrimers analyzed on reverse phase C18 columns to show hydrophobicity. (a) G5OH unmodified dendrimer (b) C1 (c) C2 (d) C3 (e) D1 (f) D2 (g) D3.
2.2. In Vitro Cell Viability
In vitro cell viability studies were performed on PLE cells isolated in our lab and also in CHO cells from ATCC, which is a commonly used cell line in studies of genetics, toxicity screening, gene expression and expression of recombinant proteins. The cell growth inhibition versus dendrimer concentration is shown in
Figure 4 for G
5OH unmodified and modified dendrimers.
Figure 4.
Cell viability curves of dendrimers in tissue culture cell lines: (a) Dodecyl modified dendrimers in PLE cells (b) Cholesteryl modified dendrimers in PLE cells (c) Dodecyl modified dendrimers in CHO cells (d) Cholesteryl modified dendrimers in CHO cells.
Figure 4.
Cell viability curves of dendrimers in tissue culture cell lines: (a) Dodecyl modified dendrimers in PLE cells (b) Cholesteryl modified dendrimers in PLE cells (c) Dodecyl modified dendrimers in CHO cells (d) Cholesteryl modified dendrimers in CHO cells.
There are several significant differences that can be noted. First, it can be seen from
Figure 4 (c), in CHO cells, the D1, D2 and D3 dendrimer IC
50 values are 1.23, 0.37, and 0.07 mg/mL, respectively. Similar results were obtained for the PLE cells [
Figure 4 (a)]. Thus, the addition of the hydrophobic chains increases toxicity in proportion to the modification as indicated by NMR as listed in
Table 1. On the other hand, the cholesteryl modified dendrimers in PLE cells
Figure 4 (b) and CHO cells
Figure 4 (d) did not show this correlation, but are all relatively similar in their response, regardless of the amount of modification. In addition, CHO cells are fairly tolerant to the unmodified G
5OH dendrimers having an IC
50 of ~25 mg/mL while the PLE cells are much less tolerant having an IC
50 of ~3 mg/mL which is almost an order of magnitude lower. The cholesteryl modified dendrimers actually showed slightly reduced toxicity when compared to the unmodified dendrimers in PLE cells, while in CHO cells the cholesteryl modified dendrimers show IC
50’s of 1-3 mg/mL. This is comparable to but slightly less tolerant than the values obtained from the PLE cells, but well below the value of 25mg/mL obtained from the unmodified G
5OH dendrimers in CHO cells.
Figure 5 shows the cell morphology of CHO cells exposed to 10 mg/mL dendrimer for 70 hours. While the C2 2% cholesteryl dendrimers do inhibit cell growth, there are very few vacuoles present, and the cells look relatively normal. The cells exposed to the D1 5% dodecyl dendrimer appear highly stressed with many vacuoles. The CHO cells exposed to the unmodified G
5OH dendrimer show less growth inhibition than cells exposed to modified dendrimers. The cells show more vacuoles than those exposed to the cholesteryl modified dendrimers but significantly less than those exposed to the dodecyl modified dendrimers.
Figure 5.
Phase contrast images showing morphology and density effects of dendrimers at 10 mg/mL on CHO cells after 70 hours incubation. (a) 2% Cholesteryl modified dendrimer (C2) (b) 5% Dodecyl modified dendrimer (D1) (c) G5OH unmodified dendrimer (d) Control, no additions.
Figure 5.
Phase contrast images showing morphology and density effects of dendrimers at 10 mg/mL on CHO cells after 70 hours incubation. (a) 2% Cholesteryl modified dendrimer (C2) (b) 5% Dodecyl modified dendrimer (D1) (c) G5OH unmodified dendrimer (d) Control, no additions.
Neutrally charged hydrophilic macromolecules are known to cross through biological barriers (cell membrane, endosomal membrane, microvessel wall, etc) depending on molecular weight or size, and geometry or architecture. However, there is ample evidence that the hydrophobic modification of dendrimers can radically affect their behavior in transport.
Figure 6 gives the structure of cholesterol and dodecane, the two hydrophobic groups used in this study. Also given as a comparison is the structure of Oregon green 488, a fluorescent compound, which was used to modify G
5NH
2 PAMAM dendrimers in another group’s study [
31]. Yoo
et al. used the modified dendrimers to transport oligonucleotides into the nuclei of HeLa cells. The results showed that the abilities as a delivery agent for the oligonucleotides were greatly enhanced (~5 fold) by the presence of the hydrophobic fluorescent label even at a 1.0:0.8 mole ratio of Oregon green 488:dendrimer. Measurements were also made confirming the dendrimers’ presence in the nuclear fractions of the cells. It was suggested that the hydrophobic fluor moieties enhance the ability of the dendrimer to disrupt endosomal membranes and thus traffic to the cytosol and nucleus.
Figure 6.
Structures of compounds. (a) Cholesterol (b) Dodecane (c) Oregon Green 488 [
24].
Figure 6.
Structures of compounds. (a) Cholesterol (b) Dodecane (c) Oregon Green 488 [
24].
Applying these observations to our study, we postulate the following, even though we have not employed an ionic dendrimer. The addition of hydrophobic elements to the surface of the dendrimers would be expected to enhance the binding of the modified dendrimer to membrane components and subsequent entrance into the cells, affecting the biocompatibility. Cholesterol is a rigid lipid that is found in abundance in cell membranes and is involved in cell metabolism. It is a molecule similar in size and hydrophobicity to the Oregon green 488 dye. Dodecane is a non-rigid hydrophobic chain. It is expected that the response to these materials would be quite different. When examining the results from the toxicities of these hydrophobized dendrimers toward the CHO and PLE cells, the most notable difference is that in the case of the dodecyl modification, there is a direct correlation between the increase in hydrophobic surface conjugation and toxicity. There was no direct relationship between toxicity of the cholesteryl dendrimers and amount of surface conjugation with cholesteryl units. With the cholesterol series, there was an initial increase in toxicity of the 1% modified dendrimer as seen in CHO cells. There was no significant difference between the 1% and 4.6% cholesterol dendrimers. In PLE cells, the cholesterol modified dendrimers were slightly less toxic than the unmodified dendrimer. Since the conditions of our MTT viability assay were comparable to those performed by Malik [
19], it is reasonable to compare our results with theirs obtained using B16F10 cells. They showed IC
50 values for G
4 amine terminated dendrimers to be in the range of 0.01 to 0.1 mg/mL, while with the anionic carboxyl terminated G
3.5 or G
7.5 dendrimers the cells did not reached the level of IC
50 toxicity at 2 mg/mL. Given these comparisons, the unmodified OH terminated dendrimers in CHO cells have similar biocompatibility to the carboxyl terminated dendrimers in biocompatibility, with an IC
50 of 25 mg/mL, while the highly substituted D3 dendrimers showed a similar toxicity to the amine terminated dendrimers (IC
50=0.07 mg/mL). It is safe to infer that the cholesterol is benign to the cells. So, evidence suggests that the toxicity of the dodecane moiety itself contributes to the reduced biocompatibility with increased concentration, though we believe that the amphiphilic character of the dendrimers increases their uptake into the cells. The difference in response between CHO and PLE cells to the G
5OH dendrimers is not clear, and will take further study to ascertain.
2.3. Dendrimer Rheology
We have previously demonstrated the possibilities of making lens refilling materials using a two component system, combining a polymer gel with nanoparticles [
34]. The viscoelastic properties of lens crystallins and G
5OH dendrimers has also been reported by our group [
37]. One main advantage to using amphiphilic materials to form the desired nanocomposites is that the hydrophobic attractions between molecules should greatly reduce the possibility of the dendrimers escaping from the nanocomposite lens material and causing damage to other ocular tissues.
Experiments were performed to evaluate the effects of the hydrophobic modification on viscoelastic properties of the modified dendrimers.
Figure 7(a) shows a frequency scan at a shear rate of 20 s
-1, which is within the linear viscoelastic response region of the material. The hydrophobic dendrimers showed an increased loss modulus over the entire frequency range, compared with the unmodified dendrimers. This is reasonable considering the increased particle size due to aggregation of the modified dendrimers.
Figure 7.
Rheological results from the Vilastic capillary rheometer. All experiments were performed at 37 °C. Dendrimers were tested at 34% w/w in PBS and lens crystallins were at physiological concentrations (~34% w/w) (a) Frequency scan at a shear rate of 20 s-1 comparing loss modulus and storage modulus of 2% cholesteryl (C2), 5% dodecyl (D1) modified and G5OH unmodified dendrimers. (b) Shear rate scan at a frequency of 2Hz, comparing the loss modulus of C2, D1 modified and G5OH unmodified dendrimers with porcine lens crystallins (c) Shear rate scan at a frequency of 2Hz, comparing the storage modulus of C2, D1 modified and G5OH unmodified dendrimers with porcine lens crystallins.
Figure 7.
Rheological results from the Vilastic capillary rheometer. All experiments were performed at 37 °C. Dendrimers were tested at 34% w/w in PBS and lens crystallins were at physiological concentrations (~34% w/w) (a) Frequency scan at a shear rate of 20 s-1 comparing loss modulus and storage modulus of 2% cholesteryl (C2), 5% dodecyl (D1) modified and G5OH unmodified dendrimers. (b) Shear rate scan at a frequency of 2Hz, comparing the loss modulus of C2, D1 modified and G5OH unmodified dendrimers with porcine lens crystallins (c) Shear rate scan at a frequency of 2Hz, comparing the storage modulus of C2, D1 modified and G5OH unmodified dendrimers with porcine lens crystallins.
Figure 7(b) and (c) show that under similar conditions, the G
5OH dendrimer at 34% w/w in addition to matching the porcine lens crystallins in refractive index and density also has a comparable storage and loss modulus. When comparing the storage modulus of the two modified dendrimers, there is one observation that stands out. The storage modulus of the 5% dodecyl dendrimer was below the sensitivity level of the instrument and could not be measured, indicating purely liquid-like behavior. This was true for all conditions of shear and frequency values tested. The storage modulus values of the 2% cholesteryl dendrimer were similar to but slightly lower than the unmodified dendrimer. If the hydrophobic dodecyl groups remain on the exterior shell of the dendrimers, the hydrophobic interaction of these groups between dendrimers should create a measurable storage modulus. The fact that we could not measure the storage modulus supports the hypothesis that the dodecyl groups could be internalized into the core of the dendrimers.
The rheology of starburst PAMAM dendrimers, was investigated by Uppuluri
et al. The results revealed that PAMAM dendrimers exhibited Newtonian flows in ethylenediamine solutions, and non-Newtonian viscoelastic response in bulk [
42,
43].
2.4. Dendrimers in Ocular Applications
Dendrimers have been used in a number of ocular applications. These applications have been reviewed by Cheng
et al. [
16]. We will mention several pertinent applications. For use in the anterior segment of the eye, Vandamme [
17] studied the use of amine, carboxylate and hydroxyl surface groups in several series of poly(amidoamine) (PAMAM) dendrimers (generations 1.5 and 2-3.5 and 4) in buffered phosphate solutions for controlled ocular drug delivery in rabbits. The duration of residence time was evaluated after solubilization of series of PAMAM dendrimers with 2 parts per thousand (w/v) of fluorescein. The New Zealand albino rabbit was used as an
in vivo model for qualitative and quantitative assessment of ocular tolerance and retention time after a single application of 25 μL of dendrimer solution to the eye. The same model was also used to determine the prolonged miotic or mydriatic activities of dendrimer solutions, some containing pilocarpine nitrate and some tropicamide, respectively. The dendrimers induced slower release of the drugs as a result of encapsulation, and exhibited some bioadhesive properties. Physiochemical parameters of the prepared eye drops suggested that the dendrimer solutions would not cause any ocular irritations. Surprisingly the amine terminated dendrimers did not appear to induce any more irritation that the hydroxyl and carboxyl terminated dendrimers.
For use in the posterior segment of the eye, Marano
et al. [
18] have synthesized polycationic lipophilic peptide core dendrimers. These agents were used to transfect human retinal pigment epithelium cells with an oligonucleotide called ODN1. This in turn was reported to cause a reduction in the production of the protein vascular endothelial growth factor. This could be used in the control of neovascularization which when out of control, can cause blindness.
Several groups are working on corneal tissue engineering materials involving dendrimers: Duan
et al. [
13] used G
2 polypropyleneimine octaamine dendrimers to generate highly crosslinked collagen with mechanical properties that would make it appropriate for use as a corneal tissue-engineering scaffold. Using carbodiimide 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC), the multifunctional amine terminated dendrimers were introduced as novel multifunctional cross-linkers, after the activation of the carboxylic acid groups of glutamic and aspartic acid residues in collagen. Young's modulus of the dendrimer cross-linked gels was in the range of 1-5 MPa. Optical transparency of the dendrimer cross-linked collagen was fairly good. Glucose permeation results demonstrated that the dendrimer cross-linked collagen had higher glucose permeability than natural human cornea. Dendrimer cross-linked collagen gels supported human corneal epithelial cell growth and adhesion, with no cell toxicity. The dendrimer cross-linked collagen gels showed promise for scaffolds for corneal tissue engineering.
Grinstaff [
19] has done work on designing and evaluating corneal adhesives prepared from “biodendrimers” using a photocrosslinking reaction and also a peptide ligation reaction to couple individual dendrimers together to form an adhesive. For example, they have synthesized a G4 poly(glycerol-succinic acid) dendrimer terminated with OH groups. Subsequent cross-linking of their dendrimers gives hydrogel adhesives. These adhesives were successfully used to repair corneal perforations; close the flaps produced in LASIK procedures, and secure corneal transplants.
The dendrimers for drug delivery do not directly apply to our application. However, these examples give indications that the above dendrimers can be used in ocular applications with minimal detrimental effects to the ocular tissues. The dendrimers cited for tissue engineering also are being used differently from the applications of the dendrimers set forth in this study in that they are employed as covalent cross-links to help form hydrogels in the MPa range. We are interested in much softer hydrogels in the 1 KPa range. It is not our intent that the hydrophobically modified dendrimers will be covalently incorporated into a hydrogel network, but rather will be kept by hydrophobic interactions in a hydrogel network formed by hydrophobically modified polymers that may not be cross-linked, or may be cross-linked at a low density. If we find that the hydrophobic interactions are insufficient to keep the nanocomposite intact, a minimum number of covalent bonds may need to be added.