Syntheses and a Solid State Structure of a Dinuclear Molybdenum(V) Complex with Pyridine
Abstract
:1. Introduction

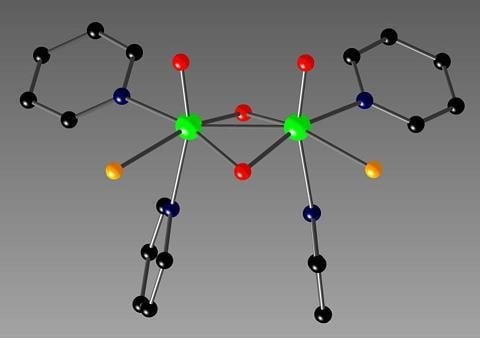
2. Results and Discussion

| 1 | 2 | |||
|---|---|---|---|---|
| Mo−Mo | 2.5502(8) | 2.5517(8) | 2.5427(6) | 2.5441(6) |
| fold angle[b] | 144.5(2) | 144.5(2) | 143.0(1) | 143.4(1) |
| Mo−Cl | 2.465(2), 2.467(2) | 2.452(2), 2.477(2) | 2.457(1), 2.460(1) | 2.453(1), 2.466(1) |
| Mo−N(Py) | 2.272(5) vs. | 2.276(5) vs. | 2.261(4) vs. | 2.256(5) vs. |
| 2.397(5) | 2.384(5) | 2.388(9) | 2.382(4) | |
| 2.265(5) vs. | 2.261(5) vs. | 2.263(4) vs. | 2.276(4) vs. | |
| 2.407(5) | 2.396(5) | 2.381(4) | 2.381(4) | |

3. Experimental Section
3.1. General
3.2. Preparation of [Mo2O4Cl2(Py)4]·2.25Py (1)
3.3. Preparation of [Mo2O4Cl2(Py)4]·1.5PyHCl (2)
3.4. X-ray crystallography
| 1 | 2 | |
|---|---|---|
| Empirical Formula | C31.25H31.25Cl2Mo2N6.25O4 | C27.5H29Cl3.5Mo2N5.5O4 |
| Formula Weight | 821.2 | 816.5 |
| Crystal System | monoclinic | monoclinic |
| Space Group | P 21/c | P 21 |
| T (K) | 153(2) | 150(2) |
| a (Å) | 17.2529(15) | 8.7374(1) |
| b (Å) | 22.279(2) | 22.4194(3) |
| c (Å) | 19.5330(18) | 16.9125(2) |
| α (deg) | 90 | 90 |
| β (deg) | 113.521(2) | 90.278(1) |
| γ (deg) | 90 | 90 |
| V (Å3) | 6884.1(11) | 3312.90(7) |
| Dcalcd (g/cm3) | 1.585 | 1.637 |
| Z | 8 | 4 |
| λ (Å) | 0.71073 | 0.71073 |
| μ (mm–1) | 0.928 | 1.080 |
| Collected Reflections | 45758 | 25375 |
| Unique Reflections, Rint | 16549, 0.1035 | 14434, 0.0243 |
| Observed Reflections | 7525 | 13356 |
| R1[a] (I>2σ(I)) | 0.0525 | 0.0286 |
| wR2[b] (all data) | 0.1230 | 0.0727 |
4. Conclusions
Acknowledgements
References
- Limberg, C.; Downs, A.J.; Blake, A.J.; Parsons, S. Modeling the formation of molybdenum oxides from alkoxides: Crystal structures of [Mo4O4Cl4(μ2-OEt)4(HOEt)2(μ3-O)2] and [PPN]+[Et3NH]+[Cl2(O)Mo(μ2-O)2Mo(O)Cl2]2−. Inorg. Chem. 1996, 35, 4439–4448. [Google Scholar] [PubMed]
- McKinney, J.D.; Chen, H.; Hamor, T.A.; Paxton, K.; Jones, C.J. Transition metal complexes containing the 1,2-dicarba-closo-dodecaborane-1,2-dithiolate ligand: Crystal structures of [4-MeC5H4NMe]2[Pd(S2C2B10H10)I2], [NEt3H][Mo(η5-C5H5)(NO)(S2C2B10H10)I], [NBu4][Re-(=O)(S2C2B10H10)2] and [4-MeC5H4NMe]2[{Mo(=O)(µ-O)(S2C2B10H10)}2]. J. Chem. Soc. Dalton Trans. 1998, 2163–2168. [Google Scholar]
- Meienberger, M.D.; Morgenstern, B.; Stucky, S.; Hegetschweiler, K. Polymerization of MoV: Synthesis and characterization of a dinuclear MoV and a hexanuclear mixed-valence MoV/MoVI complex. Eur. J. Inorg. Chem. 2008, 129–137. [Google Scholar]
- Du Peloux, C.; Dolbecq, A.; Mialane, P.; Marrot, J.; Secheresse, F. Template synthesis of {(MoV2O4)(O3PCH2PO3)}n clusters (n = 3, 4, 10): Solid state and solution studies. Dalton Trans. 2004, 1259–1263. [Google Scholar]
- Manos, M.J.; Woollins, J.D.; Slawin, A.M.Z.; Kabanos, T.A. Poyoxomolybdenum(V) sulfite complexes: Synthesis, structural, and physical studies. Angew. Chem. Int. Ed. 2002, 41, 2801–2805. [Google Scholar]
- Zhou, Z.-H.; Xu, Q.; Lin, J.; Ng, S.-W. pH dependent formations of dinuclear molybdenum(V) and incomplete cubane molybdenum(IV) complexes with nitrilotriacetate. Inorg. Chem. Commun. 2007, 10, 1461–1464. [Google Scholar]
- Zhang, Q.; Starke, K.; Schulzke, C.; Hofmeister, A.; Magull, J. Different reaction behavior of molybdenum and tungsten. Reactions of the dichloro dioxo dimethyl-bispyridine complexes with thiophenolate. Inorg. Chim. Acta 2007, 360, 3400–3407. [Google Scholar]
- Chae, H.K.; Klemperer, W.G.; Marquart, T.A. High-nuclearity oxomolybdenum(V) complexes. Coord. Chem. Rev. 1993, 128, 209–224. [Google Scholar]
- Hill, C.L. Special issue on polyoxometalates. Chem. Rev. 1998, 98, 1–390. [Google Scholar]
- Modec, B.; Brenčič, J.V. From small {Mo2O4}2+ aggregates to infinite solids. J. Cluster Sci. 2002, 13, 279–302. [Google Scholar]
- Modec, B.; Brenčič, J.V.; Golič, L.; Giester, G. Oxomolybdenum coordination compounds with pyridine. Syntheses and structures of [Mo4O8(OCH3)2Cl2L4]·2L (L = Py, 4-MePy), [Mo4O8(OCH3)4(4-MePy)4], [Mo6O12(OCH3)6Py4] and [Mo10O26Py8]·7Py. Inorg. Chim. Acta 2000, 307, 32–40. [Google Scholar]
- Modec, B.; Brenčič, J.V.; Finn, R.C.; Rarig, R.S.; Zubieta, J. Structural isomerism among octanuclear oxomolybdenum(V) coordination compounds with pyridines. Two isomers of [Mo8O16(OMe)8(R-Py)4]. Inorg. Chim. Acta 2001, 322, 113–119. [Google Scholar]
- Modec, B.; Brenčič, J.V.; Golič, L.; Daniels, L.M. Oxomolybdenum coordination compounds with alkyl-substituted pyridines. Solvothermal syntheses and structural characterization of [Mo10O26(3,5-Lut)8]·2(3,5-Lut), [Mo10O26(3-MePy)8]·(3-MePy), [Mo10O26(3-MePy)8] and [Mo12O28(OCH3)2Cl2(3-MePy)8]. Polyhedron 2000, 19, 1407–1414. [Google Scholar]
- Blake, A.J.; Downs, A.J.; Limberg, C.; Parsons, S. Synthesis and crystal structure of an encapsulated fragment of MoO2 in the unique Mo8 oxo-cluster compound [Mo8O8Cl6(µ3-O)4(OH)2(µ-OH)4(µ-OEt)4(HOEt)4] and its relevance to the sol-gel synthesis of molybdenum oxides. J. Chem. Soc., Dalton Trans. 1995, 3263–3268. [Google Scholar]
- Modec, B. En route to {Mo2O2(μ2-O)2}2+ species: Isolation and structural characterization of a novel methoxide-bridged Mo(V) complex. Inorg. Chem. Commun. 2009, 12, 328–331. [Google Scholar]
- Modec, B.; Dolenc, D.; Brenčič, J.V.; Koller, J.; Zubieta, J. Dinuclear oxomolybdate(V) species with oxalate and pyridine ligands revisited: Cis/trans isomerization of [Mo2O4(η2-C2O4)2(R-Py)2]2− (R-Py = pyridine, alkyl-substituted pyridine) in water evidenced by NMR spectroscopy. Eur. J. Inorg. Chem. 2005, 3224–3237. [Google Scholar]
- Stiefel, E.I. The coordination and bioinorganic chemistry of molybdenum. Prog. Inorg. Chem. 1977, 22, 1–223. [Google Scholar]
- Drake, J.E.; Mislankar, A.G.; Ratnani, R. Synthesis, spectroscopic characterization, and structural studies of bis(μ-sulfido)bis[{O,O-dialkyl(alkylene)dithiophosphato}oxomolybdenum(V)] complexes. Crystal structures of Mo2O2S2[S2P(OEt)2]2, Mo2O2S2[S2P(OEt)2]2·2NC5H5, and Mo2O3[S2P(OPh)2]4. Inorg. Chem. 1996, 35, 2665–2673. [Google Scholar]
- El-Essawi, M.M.; Weller, F.; Stahl, K.; Kersting, M.; Dehnicke, K. Mo2O3Cl4(Pyridine)4·CH2Cl2 - synthesis, IR spectrum, and crystal structure. Z. anorg. allg. Chem. 1986, 542, 175–181. [Google Scholar]
- The sum of the corresponding van der Waals radii is 3.3 Å. Data were taken from Douglas, B.; McDaniel, D.; Alexander, J. Concepts and Models of Inorganic Chemistry, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1994; p. 102. [Google Scholar]
- Modec, B.; Brenčič, J.V. Novel methanol-containing oxomolybdate(V) complexes: Synthesis and structural characterization of intermediates in the formation of {Mo2O4}2+ clusters from [MoOCl4(H2O)]− and [MoOBr4]− precursors. Eur. J. Inorg. Chem. 2005, 1698–1709. [Google Scholar]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97. Programs for Crystal Structure Solution and Refinement. University of Göttingen: Göttingen, The Netherlands, 1997. [Google Scholar]
- Farrugia, L.J. ORTEP-3 for Windows - a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- CrystalMaker for Windows version 2.0.; CrystalMaker Software, Ltd.: Oxfordshire, UK, 2007.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Modec, B.; Zubieta, J. Syntheses and a Solid State Structure of a Dinuclear Molybdenum(V) Complex with Pyridine. Materials 2010, 3, 150-157. https://doi.org/10.3390/ma3010150
Modec B, Zubieta J. Syntheses and a Solid State Structure of a Dinuclear Molybdenum(V) Complex with Pyridine. Materials. 2010; 3(1):150-157. https://doi.org/10.3390/ma3010150
Chicago/Turabian StyleModec, Barbara, and Jon Zubieta. 2010. "Syntheses and a Solid State Structure of a Dinuclear Molybdenum(V) Complex with Pyridine" Materials 3, no. 1: 150-157. https://doi.org/10.3390/ma3010150
APA StyleModec, B., & Zubieta, J. (2010). Syntheses and a Solid State Structure of a Dinuclear Molybdenum(V) Complex with Pyridine. Materials, 3(1), 150-157. https://doi.org/10.3390/ma3010150