Brushite-Forming Mg-, Zn- and Sr-Substituted Bone Cements for Clinical Applications
Abstract
:1. Introduction
2. Calcium Phosphate Cements
2.1. Brushite Cements
3. Incorporation of Mg, Sr and Zn Ions into TCP Structures
4. Overview of Brushite-Forming Mg-, Zn- and Sr-Substituted Cements Properties
4.1. Setting and Flow Behaviour

4.2. Injectability and Cohesion

4.3. Mechanical Strength
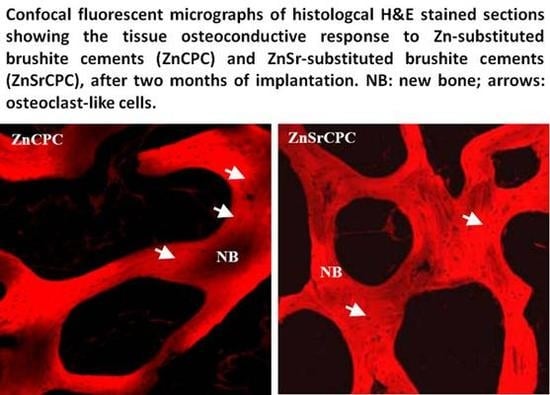
4.4. Biological Performance

5. Conclusions
Acknowledgments
References
- LeGeros, R.Z.; Daculsi, G. In vivo transformation of biphasic calcium phosphate ceramics: Ultrstructural and physicochemical characterizations. In Handbook of Bioactive Ceramics; CRC Press: Boca Raton, FL, USA, 1990; pp. 17–28. [Google Scholar]
- LeGeros, R.Z.; LeGeros, J.P. Bone Substitute Materials and Their Properties; Georg Thieme Verlag: Stuttgart/New York, Germany/USA, 1997; pp. 12–18. [Google Scholar]
- De Bruijn, J.D.; Klein, C.P.A.T.; De Groot, K. The ultrastructure of the bone-hydroxyapatite interface in vitro. J. Biomed. Mater. Res. A 1992, 26, 1365–1382. [Google Scholar] [CrossRef]
- Albee, F.H. Studies in bone growth: Triple CaP as a stimulus to osteogenesis. Ann. Surg. 1920, 71, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.D.; Ward, A.A., Jr. A preliminary report on studies of basic CaP in bone replacement. Surg. Form. 1951, 3, 429–434. [Google Scholar]
- Levitt, G.E.; Crayton, P.H.; Monroe, E.A. Forming methods for apatite prosthesis. J. Biomed. Mater. Res. 1969, 3, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Monroe, Z.A.; Votawa, W.; Base, D.B. New CaP ceramic material for bone and tooth implants. J. Dent. Res. 1971, 50, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Kato, K.; Ogiso, M.; Tabata, T. Studies on the application of apatite to dental materials. J. Dent. Eng. 1977, 18, 86–89. [Google Scholar]
- DeGroot, K. Bioceramics of CaP; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Jarcho, M. Hydroxyapatite synhesis and characterization in dense polycrystalline forms. J. Mater. Sci. 1976, 11, 2027–2035. [Google Scholar] [CrossRef]
- Metzger, D.S.; Driskell, T.D.; Paulsrud, J.R. Tricalcium phosphate ceramic: A resorbable bone implant: Review and current status. J. Amer. Dent. Assoc. 1982, 105, 1035–1048. [Google Scholar] [CrossRef]
- Nery, E.B.; Lynch, K.L.; Hirthe, W.M.; Muller, K.H. Bioceramics implants in surgically produced infrabony defects. J. Periodontol. 1975, 63, 729–735. [Google Scholar] [CrossRef]
- Nery, E.B.; Lynch, K.L. Functional loading of bioceramic augmented alveolar ridge: A pilot study. Prosthet. Dent. 1978, 43, 338–343. [Google Scholar] [CrossRef]
- Denissen, H.W. Dental root implants of apatite ceramics experimental investigations and clinical use of dental root implants made of apatite ceramics. PhD thesis, Vrije University, Amsterdam, The Netherlands, 1979. [Google Scholar]
- Denissen, H.W.; Kalk, W.; Veldhuis, A.A.; van der Hooff, A. Eleven year study of hydroxyapatite implants. J. Prosthet. Dent. 1989, 61, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Constantz, B.R.; Ison, I.C.; Fulmer, M.T.; Poser, R.D.; Smith, S.T. Coral chemistry leads to human bone repair. Science 1995, 267, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and application. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Ganeles, J.; Listgarten, M.A.; Evian, C.L. Ultrastructure of durapatite-periodontal tissue interface in human intrabony defects. J. Periodontol. 1986, 57, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Gbureck, U.; Barralet, J.E. Technological issues for the development of more efficient calcium phosphate bone cements: A critical assessment. Biomaterials 2005, 26, 6423–6429. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.; Chow, L.C. A new calcium-phosphate setting cement. J. Dent. Res. 1983, 62, 672–672. [Google Scholar]
- Gross, K.A.; Berndt, C.C. Phosphates: Geochemical, Geobiological and Materials Importance; Mineralogical Society of America: Washington DC, USA, 2002; pp. 631–672. [Google Scholar]
- Bohner, M.; Gbureck, U. Thermal reactions of brushite cements. J. Biomed. Mater. Res. B. 2008, 84, 375–385. [Google Scholar] [CrossRef]
- Hofmann, M.P.; Young, A.M.; Gbureck, U.; Nazhat, S.N.; Barralet, J.E. FTIR-monitoring of a fast setting brushite bone cement: Effect of intermediate phases. J. Mater. Chem. 2006, 16, 3199–3206. [Google Scholar] [CrossRef]
- Gbureck, U.; Dembski, S.; Thull, R.; Barralet, J.E. Factors influencing calcium phosphate cement shelf-life. Biomaterials 2005, 26, 3691–3697. [Google Scholar] [CrossRef] [PubMed]
- Constantz, B.R.; Ison, I.C.; Fulmer, M.T.; Baker, J.; McKinney, L.A.; Goodman, S.B. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J. Biomed. Mater. Res. B. 1998, 43, 451–461. [Google Scholar] [CrossRef]
- Gisep, A.; Wieling, R.; Bohner, M.; Matter, S.; Schneider, E. Resorption patterns of calcium-phosphate cements in bone. J. Biomed. Mater. Res. A 2003, 66, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Foresti, E.; Gandolfi, M.; Gazzano, M.; Roveri, N. Isomorphous substitutions in beta-tricalcium phosphate: The different effects of zinc and strontium. J. Inorg. Biochem. 1997, 66, 259–265. [Google Scholar] [CrossRef]
- Fadeev, I.V.; Shvorneva, L.I.; Barinov, S.M.; Orlovskii, V.P. Synthesis and structure of magnesium-substituted hydroxyapatite. Inorg. Mater. 2003, 39, 947–950. [Google Scholar] [CrossRef]
- Suchanek, W.; Byrappa, K.; Shuk, P.; Riman, R.; Janas, V.; TenHuisen, K.S. Mechanochemical-hydrothermal synthesis of calcium phosphate powders with coupled magnesium and carbonate substitution. J. Sol. St. Chem. 2004, 177, 793–799. [Google Scholar] [CrossRef]
- Kannan, S.; Rocha, J.H.G.; Ferreira, J.M.F. Synthesis and characterization of magnesium substituted biphasic mixtures of controlled hydroxyapatite/beta-tricalcium phosphate ratios. J. Sol. St. Chem. 2005, 178, 3190–3196. [Google Scholar] [CrossRef]
- Kannan, S.; Pina, S.; Ferreira, J.M.F. Formation of strontium-stabilized alpha-tricalcium phosphate from calcium-deficient apatite. J. Amer. Ceram. Soc. 2006, 89, 3277–3280. [Google Scholar] [CrossRef]
- Rokita, E.; Hermes, C.; Nolting, H.; Ryczek, J. Substitution of calcium by strontium within selected calcium phosphates. J. Cryst. Growth. 1993, 130, 543–552. [Google Scholar] [CrossRef]
- Li, X.; Sogo, Y.; Ito, A.; Mutsuzaki, H.; Ochiai, N.; Kobayashi, T. The optimum zinc content in set calcium phosphate cement for promoting bone formation in vivo. Mater. Sci. Eng. 2008, 29, 969–975. [Google Scholar] [CrossRef]
- Otsuka, M.; Marunaka, S.; Matsuda, Y.; Ito, A.; Layrolle, P.; Naito, H.; Ichinose, N. Calcium level-responsive in vitro zinc release from zinc-containing tricalcium phosphate (ZnTCP). J. Biomed. Mater. Res. 2000, 52, 810–824. [Google Scholar] [CrossRef]
- Marie, P.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue. Int. 2001, 69, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.G.; Allain, P.; Marie, P.J.; Mauras, Y.; Boivin, G.; Ammann, P.; Tsouderos, Y.; Delmas, P.D.; Christiansen, C. Incorporation and distribution of strontium in bone. Bone 2001, 28, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.T.; Lu, W.W.; Chan, W.K.; Cheung, K.; Luk, D.; Lu, D.; Rabie, A.; Deng, L.; Leong, J. In vivo cancellous bone remodeling on a Strontium containing hydroxyapatite (Sr-HA) bioactive cement. J. Biomed. Mater. Res. A 2003, 68, 513–521. [Google Scholar]
- Fernández, E.; Ginebra, M.; Boltong, M.G.; Driessens, F.; Ginebra, J.; De Maeyer, E.A.; Verbeeck, R.M.; Planell, J.A. Kinetic study of the setting reaction of a calcium phosphate bone cement. J. Biomed. Mater. Res. 1996, 32, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Rel. 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements: Competitive drug carriers for the musculoskeletal system? Biomaterials 2006, 27, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Inj. Intern. J. Care Inj. 2000, 31, 37–47. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates cements for biomedical application. J. Mater. Sci.: Mater. Med. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Bohner, M. Reactivity of calcium phosphate cements. J. Mater. Chem. 2007, 17, 3980–3986. [Google Scholar] [CrossRef]
- Bohner, M.; van Landuyt, P.; Merkle, H.P.; Lemaitre, J. Composition effects on the pH of a hydraulic calcium phosphate cement. J. Mater. Sci.: Mater. Med. 1997, 8, 675–681. [Google Scholar] [CrossRef]
- Duck, F.A. Physical Properties of Tissue: A Comprehensive Reference Book; Academic Press Ltd.: London, UK, 1990. [Google Scholar]
- Gbureck, U.; Barralet, J.E.; Spatz, K.; Grover, L.M.; Thull, R. Ionic dification of calcium phosphate cement viscosity. Part I: Hypodermic injection and strength improvement of apatite cement. Biomaterials 2004, 25, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Mariño, F.T.; Mastio, J.; Rueda, C.; Blanco, L.; Cabarcos, E. L.; Marino, F. T. Increase of the final setting time of brushite cements by using chondroitin 4-sulfate and silica gel. J. Mater. Sci. – Mater. Med. 2007, 18, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.T.; Torres, J.; Hamdan, M.; Rodriguez, C. R.; Cabarcos, E. L. Advantages of using glycolic acid as a retardant in a brushite forming cement. J. Biomed. Mater. Res. B – Appl. Biomater. 2007, 83, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Merkle, H.P.; Landuyt, P.V.; Trophardy, G.; Lemaitre, J. Effect of several additives and their admixtures on the physico-chemical properties of a calcium phosphate cement. J. Mater. Sci. – Mater. Med. 1999, 11, 111–116. [Google Scholar] [CrossRef]
- Alkhraisat, M.H,; Marino, F.T.; Retama, J.R.; Jerez, L.B.; Lopez-Cabarcos, E. Beta-tricalcium phosphate release from brushite cement surface. J. Biomed. Mater. Res. A 2008, 84, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Ohura, K.; Bohner, M.; Hardouin, P.; Lemaitre, J.; Pasquier, G.; Flautre, B. Resorption of, and bone formation from, new β-tricalcium phosphate cements: An In vivo study. J. Biomed. Mater. Res. B 1996, 30, 193–200. [Google Scholar] [CrossRef]
- Flautre, B.; Maynou, C.; Lemaitre, J.; Van Landuyt, P.; Hardouin, P. Bone colonization of β-TCP granules incorporated in brushite cements. J. Biomed. Mater. Res. – Appl. Biomater. 2002, 63, 413–417. [Google Scholar] [CrossRef]
- Bohner, M.; Theiss, F.; Apelt, D.; Hirsiger, W.; Houriet, R.; Rizzoli, G.; Gnos, E.; Frei, C.; Auer, J. A.; von Rechenberg, B. Compositional changes of a dicalcium phosphate dihydrate cement after implantation in sheep. Biomaterials 2003, 24, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Kumarasami, B.; Doillon, C.; Gbureck, U.; Nihouannen, D. L.; Cabarcos, E. L.; Barralet, J. E. Brushite-collagen composites for bone regeneration. Acta Biomaterialia. 2008, 4, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Ferreira, J.M.F. Synthesis and thermal stability of hydroxyapatite-beta-tricalcium phosphate composites with cosubstituted sodium, magnesium, and fluorine. Chem. Mater. 2006, 18, 198–203. [Google Scholar] [CrossRef]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Ionic substitutions in biphasic hydroxyapatite and beta-tricalcium phosphate mixtures: Structural analysis by rietveld refinement. J. Amer. Ceram. Soc. 2008, 91, 1–12. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Moseke, C.; Blanco, L.; Barralet, J.E.; Lopez-Cabarcos, E.; Gbureck, U. Strontium modified biocements with zero order release kinetics. Biomaterials 2008, 29, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Synthesis and structure refinement of zinc-doped beta-tricalcium phosphate powders. J. Amer. Ceram. Soc. 2009, 92, 1592–1595. [Google Scholar] [CrossRef]
- Pina, S.; Torres, P.M.C.; Ferreira, J.M.F. Injectability of brushite-forming Mg-substituted and Sr-substituted α-TCP bone cements. J. Mater. Sci. – Mater. Med. 2009. [Google Scholar] [CrossRef]
- Pina, S.; Torres, P.M.C.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Newly developed Sr-substituted α-TCP bone cements. Acta Biomater. 2009. [Google Scholar] [CrossRef]
- Ito, A.; Ojima, K.; Naito, H.; Ichinose, N.; Tateishi, T. Preparation, solubility, and cytocompatibility of zinc-releasing calcium phosphate ceramics. J. Biomed. Mater. Res. 2000, 50, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Alkhraisat, M.H.; Marino, F.T.; Rodriguez, C.R.; Jerez, L.B.; Cabarcos, E.L. Combined effect of strontium and pyrophosphate on the properties of brushite cements. Acta Biomater. 2008, 4, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Rebelo, A.H.S.; Valério, P.; Ferreira, J.M.F. Rietveld structure and in vitro analysis on the influence of magnesium in biphasic (hydroxyapatite and β-tricalcium phosphate) Mixtures. J. Biomed. Mater. Res.B. 2009, 90, 404–411. [Google Scholar]
- Enderle, R.; Goetz-Neunhoeffer, F.; Gobbels, M.; Muller, F. A.; Greil, P. Influence of magnesium doping on the phase transformation temperature of beta-TCP ceramics examined by Rietveld refinement. Biomaterials 2005, 26, 3379–3384. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A. Cytotoxicity and viability assays. In Animal Cell Culture: A Practical Approach; Oxford University Press: Oxford, UK, 2000; p. 207. [Google Scholar]
- Bigi, A.; Falini, G.; Foresti, E.; Ripamonti, A.; Gazzano, M.; Roveri, N. Rietveld structure refinement of synthetic magnesium substituted β-tricalcium phosphate. Z. Kristallogr. 1996, 211, 13–16. [Google Scholar] [CrossRef]
- Pina, S.; Olhero, S.M.; Gheduzzi, S.; Miles, A.W.; Ferreira, J.M.F. Influence of setting liquid composition and liquid-to-powder ratio on properties of a Mg-substituted calcium phosphate cement. Acta Biomater. 2009, 5, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- TenHuisen, K.S.; Brown, P.W. Effects of magnesium on the formation of calcium-deficient hydroxyapatite from CaHPO4 center dot 2H(2)O and Ca-4(PO4)(2)O. J. Biomed. Mater. Res. 1997, 36, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, J.; Christoffersen, M.R.; Kolthoff, N.; Barenholdt, O. Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection. Bone. 1997, 20, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F. In vitro and in vivo degradation of biomimetic octacalcium phosphate and carbonate apatite coatings on titanium implants. J. Biomed. Mater. Res. A 2003, 64, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Iwanaga, H.; Shlbutani, T.; Moriwakl, Y.; Iwayama, Y. Osteoclastic responses to various calcium phosphates in cell cultures. J. Biomed. Mater. Res. 1999, 47, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, S.; Champion, E.; Bernache-Assolant, D.; Tetard, D. Dynamic fatigue and degradation in solution of hydroxyapatite ceramics. J. Mater. Sci.: Mater. Med. 1998, 9, 221–227. [Google Scholar] [CrossRef]
- Barralet, J.; Akao, M.; Aoki, H. Dissolution of dense carbonate apatite subcutaneously implanted in Wistar rats. J. Biomed. Mater. Res. 2000, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; LeGeros, RZ; Heughebaert, M; Barbieux, I. Formation of carbonate-apatite crystals after implantation of calcium-phosphate ceramics. Calcif. Tissue. Int. 1990, 46, 20–27. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium Phosphates in Oral Biology and Medicine. In Monographs in Oral Science; Karger: New York, NY, USA, 1991. [Google Scholar]
- Alves, H.L.R.; dos Santos, L.A.; Bergmann, C.P. Injectability evaluation of tricalcium phosphate bone cement. J. Mater. Sci.: Mater. Med. 2008, 19, 2241–2246. [Google Scholar] [CrossRef]
- Del Valle, S.; Miño, N.; Muñoz, F.; González, A.; Planell, J.A.; Ginebra, M.P. In vivo evaluation of an injectable Macroporous Calcium Phosphate Cement. J. Mater. Sci. – Mater. Med. 2007, 18, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Khairoun, I.; Driessens, F.C.M.; Boltong, M.G.; Planell, J.A.; Wenz, R. Addition of cohesion promoters to calcium phosphate cements. Biomaterials 1999, 20, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Alkhraisat, M.H.; Rueda, C.; Jerez, L.B.; Marino, F.T.; Torres, J.; Gbureck, U.; Cabarcos, E.L. Effect of silica gel on the cohesion, properties and biological performance of brushite cement. Acta Biomater. 2010, 6, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Barralet, J.; Tremayne, M.; Lilley, K.; Gbureck, U. Modification of calcium phosphate cement with α-hydroxy acids and their salts. Chem. Mater. 2005, 17, 1313–1319. [Google Scholar] [CrossRef]
- Bohner, M.; Baroud, G. Injectability of calcium phosphate pastes. Biomaterials 2005, 26, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Takagi, S.; Chow, L.C.; Ishikawa, Y. Properties and mechanisms of fast-setting calcium phosphate cements. J. Mater. Sci.: Mater. Med. 1995, 6, 528–533. [Google Scholar] [CrossRef]
- Driessens, F.C.M.; Boltong, M.G.; De Maeyer, E.A.P.; Verbeeck, R.M.H.; Wenz, R. Effect of temperature and immersion on the setting of some calcium phosphate cements. J. Mater. Sci.: Mater. Med. 2000, 11, 453–457. [Google Scholar] [CrossRef]
- Ikenaga, M.; Hardouin, P.; Lemaitre, J.; Andrianjatovo, H.; Flautre, B. Biomechanical characterization of a biodegradable calcium phosphate hydraulic cement: A comparison with porous biphasic calcium phosphate ceramics. J. Biomed. Mater. Res. 1998, 40, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Ishikawa, K.; Fukao, H.; Sawada, M.; Nagayama, M.; Kon, M.; Asaoka, K. In vivo setting behavior of fast-setting calcium-phosphate cement. Biomaterials 1995, 16, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Lilley, K.J.; Gbureck, U.; Knowles, J.C.; Farrar, D.F.; Barralet, J.E. Cement from magnesium substituted hydroxyapatite. J. Mater. Sci.: Mater. Med. 2005, 16, 455–460. [Google Scholar] [CrossRef]
- Klammert, U.; Reuther, T.; Blank, M.; Reske, I.; Barralet, J.; Grover, L.M.; Kluber, A.C.; Gbureck, U. Phase composition, mechanical performance and in vitro biocompatibility of hydraulic setting calcium magnesium phosphate cement. Acta Biomater. 2009. [Google Scholar] [CrossRef]
- Apelt, D.; Theiss, F.; El-Warrak, A.O.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.A.; von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Ooms, E.M.; Wolke, J.G.; van de Heuvel, M.T.; Jeschke, B.; Jansen, J.A. Histological evaluation of the bone response to calcium phosphate cement implanted in cortical bone. Biomaterials 2003, 24, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z.; LeGeros, J.P. Calcium phosphate bioceramics: past, present and future. Key Eng. Mater. 2003, 240-242, 3–10. [Google Scholar] [CrossRef]
- Schliephake, H.; Gruber, R.; Dard, M.; Wenz, R.; Scholz, S. Repair of calvarial defects in rats by prefabricated hydroxyapatite cement implants. J. Biomed. Mater. Res. A 2004, 69, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.P.; Li, Y.B.; de Bruijn, J.D.; de Groot, K.; Zhang, X.D. Tissue responses of calcium phosphate cement: A study in dogs. Biomaterials 2000, 21, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Arthur, E. Physiological functions of calcium, magnesium and mineral ion balance. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Murray, J., Ed.; Lippincott-Raven: New York, NY, USA, 1993; pp. 41–46. [Google Scholar]
- Eisman, J. Pathogenesis of osteoporosis. In Rheumatology; Kippel, J., Dieppe, P., Eds.; Mosby: London, UK, 1998; p. 8. [Google Scholar]
- Wallach, S. Relation of magnesium to osteoporosis and calcium urolithiasis. Magnes Trace Elem. 1991-1992, 10, 281–286. [Google Scholar]
- Dahl, S.; Allain, P.; Marie, P.J. Incorporation and distribution of strontium in bone. Bone 2001, 28, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Ohshita, Y.; Marunaka, S.; Matsuda, Y.; Ito, A.; Ichinose, N.; Otsuka, K.; Higuchi, W.I. Effect of controlled zinc release on bone mineral density from injectable Zn-containing beta-tricalcium phosphate suspension in zinc-deficient diseased rats. J. Biomed. Mater. Res. A 2004, 69, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.; Falchuk, K. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 72, 79–118. [Google Scholar]
- O’Dell, B. Zinc plays both structural and catalytic roles in metalloproteins. Nutr. Rev. 1992, 50, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.; Wadiwala, I.; Sharland, D.; Rai, G. Copper and zinc levels in health and sick elderly. J. Amer. Geriatr. Soc. 1985, 33, 847–849. [Google Scholar]
- Pina, S. Cements of Doped Calcium Phosphates for Bone Implantation. PhD Thesis, University of Aveiro, Aveiro, Portugal, 2009. [Google Scholar]
- Johal, K.K.; Hill, R.G.; Brook, I.M. In vivo response of strontium and zinc based ionomeric cement implants in bone. J. Mater. Sci.: Mater. Med. 2002, 13, 543–552. [Google Scholar] [CrossRef]
- Alkraisat, M.H.; Rueda, C.; Cabrejos-Azama, J.; Lucas-Aparício, J.; Marino, F.T.; Garcia-Denche, J.T.; et al. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater. 2009. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S.; Sandri, M.; Logroscino, G. Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomater. 2007, 3, 961–999. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pina, S.; Ferreira, J.M.F. Brushite-Forming Mg-, Zn- and Sr-Substituted Bone Cements for Clinical Applications. Materials 2010, 3, 519-535. https://doi.org/10.3390/ma3010519
Pina S, Ferreira JMF. Brushite-Forming Mg-, Zn- and Sr-Substituted Bone Cements for Clinical Applications. Materials. 2010; 3(1):519-535. https://doi.org/10.3390/ma3010519
Chicago/Turabian StylePina, Sandra, and José M.F. Ferreira. 2010. "Brushite-Forming Mg-, Zn- and Sr-Substituted Bone Cements for Clinical Applications" Materials 3, no. 1: 519-535. https://doi.org/10.3390/ma3010519
APA StylePina, S., & Ferreira, J. M. F. (2010). Brushite-Forming Mg-, Zn- and Sr-Substituted Bone Cements for Clinical Applications. Materials, 3(1), 519-535. https://doi.org/10.3390/ma3010519