Cell-Based Fabrication of Organic/Inorganic Composite Gel Material
Abstract
:1. Introduction
2. Results and Discussion
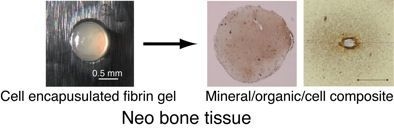
2.1. BMSCs Culture in 3D Fibrin Gel

2.2. Deposition and Distribution of Matrix and Minerals in Fibrin Gel






2.3. Composition of Obtained 3D Constructs

2.4. Osteoconductivity of Synthesized Materials

3. Experimental Section
3.1. BMSCs Culture in Fibrin Gel
3.2. Deposition and Distribution of Matrix and Minerals in Fibrin Gel
3.3. Image Analysis
- (1)
- Color information expressed in RGB color was obtained from each stained image (HE or von Kossa).
- (2)
- A mineral (X) in the imaged area was defined as the region where both blue/green < 0.85 and red/green >1.02 were acquired in the von Kossa-stained samples. A matrix (Y) was defined as the region where 210 < red < 225 was acquired in the HE-stained samples. A cell (Z) was fixed as 1.5% in volume because our previous work indicated that proliferation of myoblasts or osteoblasts in 3D fibrin gel slightly increased at the start of culture and saturated by day 5 and that the number of cells was maintained following the culture period [31].
- (3)
- The volume ratio of each component was expressed as the following equation.Vx = Mineral(X)/f, Vy = Matrix(Y)/f, Vz = 1.5/fFibrin gel = 100 × (1 − Vx − Vy − Vz)f = {initial gel volume—gel volume (day 42)}/42
3.4. Osteoconductivity of Synthesized Materials
3.5. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Salter, R.B. Normal structure and function of musculoskeletal tissues. In Textbook of Disorders and Injuries of the Musculoskeletal System; LWW: Philadelphia, PA, USA, 1999; p. 7. [Google Scholar]
- Suzuki, N.; Ohneda, O.; Minegishi, N.; Nishikawa, M.; Ohta, T.; Takahashi, S.; Engel, J.D.; Yamamoto, M. Combinatorial Gata2 and Sca1 expression defines hematopoietic stem cells in the bone marrow niche. Proc. Natl. Acad. Sci. USA 2006, 103, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.A. Re-treatment after full-course radiotherapy. Acta Oncol. 1999, 38, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Novince, C.M.; Ward, B.B.; McCauley, L.K. Osteonecrosis of the jaw: An update and review of recommendations. Cells Tissues Organs 2009, 189, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yamaguchi, Y.; Tsuji, M.; Shigematsu, M.; Goto, M. Mandibular reconstruction using autologous iliac bone and titanium mesh reinforced by laser welding for implant placement. Int. J. Oral Maxillofac. Implants 2008, 23, 1143–1146. [Google Scholar] [PubMed]
- Sotereanos, N.G.; DeMeo, P.J.; Hughes, T.B.; Bargiotas, K.; Wohlrab, D. Autogenous osteochondral transfer in the femoral head after osteonecrosis. Orthopedics 2008, 31, 177. [Google Scholar] [CrossRef] [PubMed]
- Van Blitterswijk, C.A.; Grote, J.J.; Kuijpers, W.; Daems, W.T.; de Groot, K. Macropore tissue ingrowth: A quantitative and qualitative study on hydroxyapatite ceramic. Biomaterials 1986, 7, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Kaigler, D.; Rice, K.G.; Krebsbach, P.H.; Mooney, D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J. Bone Miner Res. 2005, 20, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.S.; Lee, S.H.; Eun Ahn, S.; Gwak, S.J.; Song, J.H.; Kim, B.S.; Chung, H.M. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly(d,l-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds. Biomaterials 2008, 29, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Mirmalek-Sani, S.H.; Hanley, N.A.; Ivanov, A.L.; Barry, J.J.; Upton, C.; Shakesheff, K.M.; Howdle, S.M.; Antonov, E.N.; Bagratashvili, V.N.; Popov, V.K.; Oreffo, R.O. Biocompatibility and osteogenic potential of human fetal femur-derived cells on surface selective laser sintered scaffolds. Acta Biomater. 2009, 5, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Anada, T.; Honda, Y.; Kamakura, S.; Matsui, K.; Matsui, A.; Sasaki, K.; Morimoto, S.; Echigo, S.; Suzuki, O. Synthetic octacalcium phosphate augments bone regeneration correlated with Its content in collagen scaffold. Tissue Eng. Part A 2009, 15, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Okada, K.; Takano, Y.; Hiraishi, Y.; Okita, Y. Autologous fibrin-coated small-caliber vascular prostheses improve antithrombogenicity by reducing immunologic response. J. Thorac. Cardiovasc. Surg. 2007, 133, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, H.P.; Meyer, U.; Plate, U.; Höhling, H.J. Aspects of collagen mineralization in hard tissue formation. Int. Rev. Cytol. 2005, 242, 121–156. [Google Scholar] [PubMed]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [PubMed]
- Barocas, V.H.; Tranquillo, R.T. An anisotropic biphasic theory of tissue-equivalent mechanics: The interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J. Biomech. Eng. 1997, 119, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Barocas, V.H.; Moon, A.G.; Tranquillo, R.T. The fibroblast-populated collagen microsphere assay of cell traction force—Part 2: Measurement of the cell traction parameter. J. Biomech. Eng. 1995, 117, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit. Rev. Oral. Biol. Med. 1999, 10, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Vattikuti, R.; Towler, D.A. Osteogenic regulation of vascular calcification: an early perspective. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E686–E696. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Mou, C.Y.; Chan, J.C. Solid-state NMR study of the transformation of octacalcium phosphate to hydroxyapatite. J. Am. Chem. Soc. 2006, 128, 6909–6918. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Moriwaki, Y.; Wen, H.B.; Fincham, A.G.; Moradian-Oldak, J. Elongated growth of octacalcium phosphate crystals in recombinant amelogenin gels under controlled ionic flow. J. Dent. Res. 2002, 81, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Smerdel-Ramoya, A.; Zanotti, S.; Deregowski, V.; Canalis, E. Connective tissue growth factor enhances osteoblastogenesis in vitro. J. Biol. Chem. 2008, 283, 22690–22699. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yoshiko, Y.; Yamamoto, R.; Minamizaki, T.; Kozai, K.; Tanne, K.; Aubin, J.E.; Maeda, N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J. Bone Miner Res. 2008, 23, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Kirshner, J.; Chen, C.J.; Liu, P.; Huang, J.; Shively, J.E. CEACAM1-4S, a cell-cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc. Natl. Acad. Sci. USA 2003, 100, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, P. FEM-based oxygen consumption and cell viability models for avascular pancreatic islets. Theor. Biol. Med. Model 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama, S.E.; Schense, J.C.; Hubbell, J.A. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: An example of designer matrices in tissue engineering. FASEB J. 1999, 13, 2214–2224. [Google Scholar] [PubMed]
- Arrighi, I.; Mark, S.; Alvisi, M.; Von Rechenberg, B.; Hubbell, J.A.; Schense, J.C. Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials 2009, 30, 1763–1771. [Google Scholar] [PubMed]
- Matsumoto, T.; Yung, Y.C.; Fischbach, C.; Kong, H.J.; Nakaoka, R.; Mooney, D.J. Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng. 2007, 13, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Yoh, R.; Matsumoto, T.; Sasaki, J.; Sohmura, T. Biomimetic fabrication of fibrin/apatite composite material. J. Biomed. Mater. Res. A 2008, 87, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sasaki, J.; Alsberg, E.; Egusa, H.; Yatani, H.; Sohmura, T. Three-dimensional cell and tissue patterning in a strained fibrin gel system. PLoS One 2007, 2, e1211. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matsumoto, T.; Mizuno, A.; Kashiwagi, M.; Yoshida, S.-s.; Sasaki, J.-i.; Nakano, T. Cell-Based Fabrication of Organic/Inorganic Composite Gel Material. Materials 2011, 4, 327-338. https://doi.org/10.3390/ma4010327
Matsumoto T, Mizuno A, Kashiwagi M, Yoshida S-s, Sasaki J-i, Nakano T. Cell-Based Fabrication of Organic/Inorganic Composite Gel Material. Materials. 2011; 4(1):327-338. https://doi.org/10.3390/ma4010327
Chicago/Turabian StyleMatsumoto, Takuya, Ami Mizuno, Miki Kashiwagi, Shin-suke Yoshida, Jun-ichi Sasaki, and Takayoshi Nakano. 2011. "Cell-Based Fabrication of Organic/Inorganic Composite Gel Material" Materials 4, no. 1: 327-338. https://doi.org/10.3390/ma4010327
APA StyleMatsumoto, T., Mizuno, A., Kashiwagi, M., Yoshida, S. -s., Sasaki, J. -i., & Nakano, T. (2011). Cell-Based Fabrication of Organic/Inorganic Composite Gel Material. Materials, 4(1), 327-338. https://doi.org/10.3390/ma4010327