Enhanced Photocatalytic Degradation of Methyl Orange Dye under the Daylight Irradiation over CN-TiO2 Modified with OMS-2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Properties of CN-TiO2/OMS-2 and Reference Samples

| Samples | SBET(m2·g−1) a | Pore volume (cm3·g−1) | Crystal phase | Crystal size (nm) b |
|---|---|---|---|---|
| CN-TiO2-400 | 40.1 | 0.074 | Anatase | 16.5 |
| CN-TiO2/OMS-2-400 | 114.7 | 0.252 | Anatase | 17.0 |
| CN-TiO2/OMS-2-500 | 79.5 | 0.278 | Anatase | 21.8 |
| CN-TiO2/OMS-2-600 | 21.9 | 0.187 | Anatase+Rutile | 22.9 |




2.2. Photocatalytic Activity of CN-TiO2/OMS-2 Samples

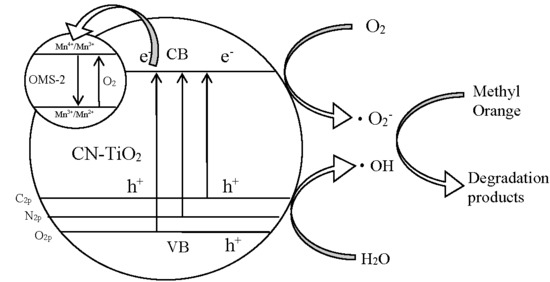
2.3. Photocatalytic Mechanism

3. Experimental Section
3.1. Chemicals
3.2. Synthesis of Daylight-Activated CN-TiO2/OMS-2 Samples
3.3. Characterization of Synthesized CN-TiO2/OMS-2 Samples
3.4. Photocatalytic Evaluation with Methyl orange under Daylight
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akhavan, O.; Azimirad, R. Photocatalytic property of Fe2O3 nanograin chains coated by TiO2 nanolayer in visible light irradiation. Appl. Catal. A Gen. 2009, 369, 77–82. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedra with exposed {001} facets. J. Am. Chem. Soc. 2010, 132, 11914–11916. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.G.; Kottam, N.; Kumar, S.G. Preparation and characterization of Mn-doped titanates with a bicrystalline framework: Correlation of the crystallite size with the synergistic effect on the photocatalytic activity. J. Phys. Chem. C 2009, 113, 15593–15601. [Google Scholar] [CrossRef]
- Wen, C.Z.; Jiang, H.B.; Qiao, S.Z.; Yang, H.G.; Lu, G.Q. Synthesis of high-reactive facets dominated anatase TiO2. J. Mater. Chem. 2011, 21, 7052–7061. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Wang, W.G.; Jaroniec, M. Nitrogen self-doped nanosized TiO2 sheets with exposed {001} facets for enhanced visible-light photocatalytic activity. Chem. Commun. 2011, 47, 6906–6908. [Google Scholar] [CrossRef]
- Yu, J.G.; Xiang, Q.J.; Zhou, M.H. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first principles study for electronic structures. Appl. Catal. B Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Zhan, S.; Yang, Y.; Gao, X.; Yu, H.; Yang, S.; Zhu, D.; Li, Y. Rapid degradation of toxic toluene using novel mesoporous SiO2 doped TiO2 nanofibers. Catal. Today 2014, 225, 10–17. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Erickson, L.; Hohn, K.; Maghirang, R.; Klabunde, K. Photo-catalytic degradation of Rhodamine B on C-, S-, N-, and Fe-doped TiO2 under visible-light irradiation. Appl. Catal. B Environ. 2009, 91, 657–662. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Seristatidou, E.; Deligiannakis, Y.; Konstantinou, I. Photocatalytic activity of N-doped and N–F co-doped TiO2 and reduction of chromium (VI) in aqueous solution: An EPR study. Appl. Catal. B Environ. 2013, 132, 460–468. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Y.; Lu, C.; Xu, Z. Hydrogenation Temperature related inner structures and visible-light-driven photocatalysis of N–F co-doped TiO2 nanosheets. Appl. Surf. Sci. 2014, 290, 125–130. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Cheng, B.; Zhao, X.; Mol, J. Preparation, characterization and photocatalytic activity of in situ N,S-codoped TiO2 powders. Catal. A Chem. 2006, 246, 176–184. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; He, T.; Guo, X.; Feng, Y. Preparation and photocatalytic activity of B–N co-doped mesoporous TiO2. Powder Technol. 2014, 253, 608–613. [Google Scholar] [CrossRef]
- Xu, Q.C.; Wellia, D.V.; Yan, S.; Liao, D.W.; Lim, T.M.; Tan, T.T.Y. Enhanced photocatalytic activity of C–N-codoped TiO2 films prepared via an organic-free approach. J. Hazard. Mater. 2011, 188, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.C.; Wellia, D.V.; Sk, M.A.; Lim, K.H.; Loo, J.S.C.; Liao, D.W.; Amal, R.; Tan, T.T.Y. Transparent visible light activated C–N–F-codoped TiO2 films for self-cleaning applications. J. Photochem. Photobiol. A Chem. 2010, 210, 181–187. [Google Scholar] [CrossRef]
- Wang, X.; Lim, T. Solvothermal synthesis of C–N codoped TiO2 and photocatalytic evaluation for bisphenol A degradation using a visible-light irradiated LED photoreactor. Appl. Catal. B Environ. 2010, 100, 355–364. [Google Scholar] [CrossRef]
- Wang, X.; Lim, T. Effect of hexamethylenetetramine on the visible-light photocatalytic activity of C–N codoped TiO2 for bisphenol A degradation: evaluation of photocatalytic mechanism and solution toxicity. Appl. Catal. A Gen. 2011, 399, 233–241. [Google Scholar] [CrossRef]
- Kakroudi, M.A.; Kazemi, F.; Kaboudin, B. Highly efficient photodeoximation under green and blue LEDs catalyzed by mesoporous C–N codoped nano TiO2. J. Mol. Catal. A Chem. 2014, 392, 112–119. [Google Scholar] [CrossRef]
- Liu, G.; Han, C.; Pelaez, M.; Zhu, D.; Liao, S.; Likodimos, V.; Kontos, A.G.; Falaras, P.; Dionysiou, D.D. Enhanced visible light photocatalytic activity of C–N-codoped TiO2 films for the degradation of microcystin-LR. J. Mol. Catal. A Chem. 2013, 373, 58–65. [Google Scholar] [CrossRef]
- Xu, L.; Steinmiller, M.P.; Skrabalak, S.E. Achieving synergy with a potential photocatalytic Z-scheme: Synthesis and evaluation of nitrogen-doped TiO2/SnO2 composites. J. Phys. Chem. C 2012, 116, 871–877. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Kumar, R.; Kanjilal, D. Dense electronic excitation induced modification in TiO2 doped SnO2 nanocomposite films. J. Alloy. Compd. 2014, 610, 651–658. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, S.; Chen, X.; Tang, Y.; Jiang, Y.; Peng, Z.; Wang, H. One-step template-free fabrication of mesoporous ZnO/TiO2 hollow microspheres with enhanced photocatalytic activity. Appl. Surf. Sci. 2014, 307, 263–271. [Google Scholar] [CrossRef]
- Pozan, G.S.; Kambur, A. Significant enhancement of photocatalytic activity over bifunctional ZnO–TiO2 catalysts for 4-chlorophenol degradation. Chemosphere 2014, 105, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Hu, T.; Zhang, W.; Zhang, X.; Chen, X. Preparation of highly photocatalytic active CdS/TiO2 nanocomposites by combining chemical bath deposition and microwave-assisted hydrothermal synthesis. J. Solid State Chem. 2014, 218, 81–89. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Song, W.; Wang, X.; Di, W.; Qin, W. CdS embedded TiO2 hybrid nanospheres for visible light photocatalysis. J. Mol. Catal. A Chem. 2014, 387, 1–6. [Google Scholar] [CrossRef]
- Song, G.; Xin, F.; Chen, J.; Yin, X. Photocatalytic reduction of CO2 in cyclohexanol on CdS–TiO2 heterostructured photocatalyst. Appl. Catal. A Gen. 2014, 473, 90–95. [Google Scholar] [CrossRef]
- Murgolo, S.; Petronella, F.; Ciannarella, R.; Comparelli, R.; Agostiano, A.; Curri, M.L.; Mascolo, G. UV and solar-based photocatalytic degradation of organic pollutants by nano-sized TiO2 grown on carbon nanotubes. Catal. Today 2015, 240, 114–124. [Google Scholar] [CrossRef]
- Pang, D.; Wang, Y.; Ma, X.; Ouyang, F. Fluorine promoted and silica supported TiO2 for photocatalytic decomposition of acrylonitrile under simulant solar light irradiation. Chem. Eng. J. 2014, 258, 43–50. [Google Scholar] [CrossRef]
- Polat, M.; Soylu, A.M.; Erdogan, D.A.; Erguven, H.; Vovk, E.I.; Ozensoy, E. Influence of the sol–gel preparation method on the photocatalytic NO oxidation performance of TiO2/Al2O3 binary oxides. Catal. Today 2015, 241, 25–32. [Google Scholar] [CrossRef]
- Pan, Z.; Stemmler, E.A.; Cho, H.J.; Fan, W.; LeBlanc, L.A.; Patterson, H.H.; Amirbahman, A. Photocatalytic degradation of 17α-ethinylestradiol (EE2) in the presence of TiO2-doped zeolite. J. Hazard. Mater. 2014, 279, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Suib, S.L. Sorption, catalysis, separation design. Chem. Innovat. 2000, 30, 27–33. [Google Scholar]
- Suib, S.L. Structure, porosity, and redox in porous manganese oxide octahedral layer and molecular sieve materials. J. Mater. Chem. 2008, 18, 1623–1631. [Google Scholar] [CrossRef]
- Abecassis-Wolfovich, M.; Jothiramalingam, R.; Landau, M.V.; Herskowitz, M.; Viswanathan, B.; Varadarajan, T.K. Cerium incorporated ordered manganese oxide OMS-2 materials: Improved catalysts for wet oxidation of phenol compounds. Appl. Catal. B Environ. 2005, 59, 91–98. [Google Scholar] [CrossRef]
- Alvarez-Lemus, M.; López, T.; Recillas, S.; Frías, D.M.; Montes, M.; Delgado, J.J.; Centeno, M.A.; Odriozola, J.A. Photocatalytic degradation of 2, dichlorophenoxyacetic acid using nanocrystalline cryptomelane composite catalysts. J. Mol. Catal. A Chem. 2008, 281, 107–112. [Google Scholar] [CrossRef]
- Liu, G.; Liao, S.; Zhu, D.; Cui, J.; Zhou, W. Solid-phase photocatalytic degradation of polyethylene film with manganese oxide. Solid State Sci. 2011, 46, 1290–1295. [Google Scholar]
- Yeung, K.L.; Yau, S.T.; Maira, A.J.; Coronado, J.M.; Soria, J.; Yue, P.L. The influence of surface properties on photocatalytic activity of nanostructured TiO2. J. Catal 2003, 219, 107–116. [Google Scholar] [CrossRef]
- Lu, X.; Song, C.; Jia, S.; Tong, Z.; Tang, X.; Teng, Y. Low-temperature selective catalytic reduction of NOx with NH3 over cerium and manganese oxides supported on TiO2–graphene. Chem. Eng. J. 2015, 260, 776–784. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, W.; Lee, S.; Zhang, L.; Li, G.; Jimmy, C.Y. Effect of carbon doping on the mesoporous structure of nanocrystalline tita-nium dioxide and its solar-light-driven photocatalytic degradation of NOx. Langmuir 2008, 24, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.G.; Yu, J.C.; Leung, M.K.P.; Ho, W.K.; Cheng, B.; Zhao, X.J.; Zhao, J.C. Effects of acidic and basic hydrolysis catalysts on the photocatalytic activity and microstructures of bimodal mesoporous titania. J. Catal. 2003, 217, 69–78. [Google Scholar]
- Sun, H.; Bai, Y.; Liu, H.; Jin, W.; Xu, N.; Chen, G.; Xu, B. Mechanism of nitrogen-concentration dependence on pH value: Experimental and theoretical studies on nitrogen-doped TiO2. J. Phys. Chem. 2008, 112, 13304–13309. [Google Scholar]
- Zhang, F.; Zhao, J.; Shen, T.; Hidaka, H.; Pelizzetti, E.; Serpone, N. TiO2-assisted photodegradation of dye pollutants II. Adsorption and degradation kinetics of eosin in TiO2 dispersions under visible light irradiation. Appl. Catal. B Enivon. 1998, 15, 147–156. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.E.; Chen, J.; Liu, G.; Zhu, D.; Cai, J. Enhanced Photocatalytic Degradation of Methyl Orange Dye under the Daylight Irradiation over CN-TiO2 Modified with OMS-2. Materials 2014, 7, 8024-8036. https://doi.org/10.3390/ma7128024
Hassan ME, Chen J, Liu G, Zhu D, Cai J. Enhanced Photocatalytic Degradation of Methyl Orange Dye under the Daylight Irradiation over CN-TiO2 Modified with OMS-2. Materials. 2014; 7(12):8024-8036. https://doi.org/10.3390/ma7128024
Chicago/Turabian StyleHassan, Mohamed Elfatih, Jing Chen, Guanglong Liu, Duanwei Zhu, and Jianbo Cai. 2014. "Enhanced Photocatalytic Degradation of Methyl Orange Dye under the Daylight Irradiation over CN-TiO2 Modified with OMS-2" Materials 7, no. 12: 8024-8036. https://doi.org/10.3390/ma7128024
APA StyleHassan, M. E., Chen, J., Liu, G., Zhu, D., & Cai, J. (2014). Enhanced Photocatalytic Degradation of Methyl Orange Dye under the Daylight Irradiation over CN-TiO2 Modified with OMS-2. Materials, 7(12), 8024-8036. https://doi.org/10.3390/ma7128024