Accumulation and Translocation of Phosphorus, Calcium, Magnesium, and Aluminum in Pinus massoniana Lamb. Seedlings Inoculated with Laccaria bicolor Growing in an Acidic Yellow Soil
Abstract
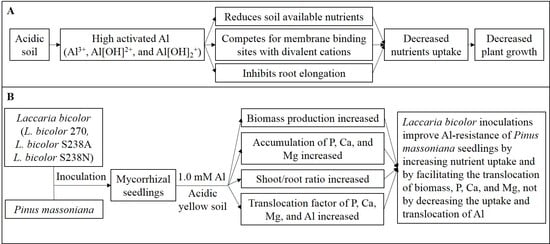
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Fungal Isolates and Production of ECM Plant Seedlings
2.3. Aluminum Treatment
2.4. Sampling and Analysis
2.5. Statistical Analysis
3. Results
3.1. Biomass of P. massoniana Seedlings
3.2. Accumulation of P, Ca, Mg, and Al in P. massoniana Seedlings
3.3. Shoot/Root Biomass Ratio in P. massoniana Seedlings
3.4. Translocation of P, Ca, Mg, and Al in P. massoniana Seedlings
3.5. Contributions of P, Ca, Mg, and Al to the Growth in P. massoniana Seedlings
4. Discussion
4.1. Aluminum Resistance and Biomass Production in ECM P. massoniana Seedlings
4.2. Accumulation and Translocation of P, Ca, and Mg in ECM P. massoniana Seedlings
4.3. Accumulation and Translocation of Al in ECM P. massoniana Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moustaka, J.; Ouzounidou, G.; Baycu, G.; Moustakas, M. Aluminum resistance in wheat involves maintenance of leaf Ca2+ and Mg2+ content, decreased lipid peroxidation and Al accumulation, and low photosystem II excitation pressure. BioMetals 2016, 29, 611–623. [Google Scholar] [CrossRef]
- Han, G.; Deng, X.; Jiang, C.; Gu, X. The research progress on the cause and control of aluminum toxicity to plants. J. Fujian For. Sci. Technol. 2007, 34, 174–179. [Google Scholar]
- Hirano, Y.; Isomura, A.; Kaneko, S. Root morphology and nutritional status of Japanese red cedar saplings subjected to in situ levels of aluminum in forest soil solution. J. For. Res. 2003, 8, 209–214. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Gunsé, B.; Corrales, I.; Barceló, J. A glance into aluminum toxicity and resistance in plants. Sci. Total Environ. 2008, 400, 356–368. [Google Scholar] [CrossRef]
- Godbold, D.L.; Fritz, E.; Hüttermann, A. Aluminum toxicity and forest decline. Proc. Natl. Acad. Sci. USA 1988, 85, 3888–3892. [Google Scholar] [CrossRef] [Green Version]
- Scholl, L.; Keltjens, W.G.; Hoffland, E.; Breemen, N. Effect of ectomycorrhizal colonization on the uptake of Ca, Mg and Al by Pinus sylvestris under aluminium toxicity. For. Ecol. Manag. 2005, 215, 352–360. [Google Scholar] [CrossRef]
- Ray, P.; Tiwari, R.; Reddy, U.G.; Adholeya, A. Detecting the heavy metal tolerance level in ectomycorrhizal fungi in vitro. World J. Microbiol. Biotechnol. 2005, 21, 309–315. [Google Scholar] [CrossRef]
- Egerton-Warburton, L. Aluminum-tolerant Pisolithus ectomycorrhizas confer increased growth, mineral nutrition, and metal tolerance to Eucalyptus in acidic mine spoil. Appl. Environ. Soil Sci. 2015, 2015, 803821. [Google Scholar] [CrossRef] [Green Version]
- Tahara, K.; Norisada, M.; Tange, T.; Yagi, H.; Kojima, K. Ectomycorrhizal association enhances Al tolerance by inducing citrate secretion in Pinus densiflora. Soil Sci. Plant Nutr. 2005, 51, 397–403. [Google Scholar] [CrossRef]
- Hentschel, E.; Godbold, D.L.; Marschner, P.; Schlegel, H.; Jentschke, G. The effect of Paxillus involutus Fr. on aluminum sensitivity of Norway spruce seedlings. Tree Physiol. 1993, 12, 379–390. [Google Scholar] [CrossRef]
- Khosla, B.; Kaur, H.; Reddy, M.S. Influence of ectomycorrhizal colonization on the growth and mineral nutrition of Populus deltoides under Aluminum toxicity. J. Plant Interact. 2009, 4, 93–99. [Google Scholar] [CrossRef]
- Gu, X.; Liang, G.; Huang, J. Mechanism on increasing plant aluminum resistance by ectomycorrhizae. Chin. Agric. Sci. Bull. 2005, 21, 218–221. [Google Scholar]
- Desai, S.; Naik, D.; Cumming, J.R. The influence of phosphorus availability and Laccaria bicolor symbiosis on phosphate acquisition, antioxidant enzyme activity, and rhizosphere carbon flux in Populus tremuloides. Micorrhiza 2014, 24, 369–382. [Google Scholar] [CrossRef]
- Scholl, L.; Kuyper, T.W.; Smits, M.M.; Landeweert, R.; Hoffland, E.; Breemen, N. Rock-eating mycorrhizas: Their role in plant nutrition and biogeochemical cycles. Plant Soil 2008, 303, 35–47. [Google Scholar] [CrossRef]
- Remiszewski, K.A.; Bryce, J.G.; Fahnestock, M.F.; Pettitt, E.A.; Blichert-Toft, J.; Vadeboncoeur, M.A.; Bailey, S.W. Elemental and isotopic perspectives on the impact of arbuscular mycorrhizal and ectomycorrhizal fungi on mineral weathering across imposed geologic gradients. Chem. Geol. 2016, 445, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Bellion, M.; Courbot, M.; Jacob, C.; Blaudez, D.; Chalot, M. Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol. Lett. 2006, 254, 173–181. [Google Scholar] [CrossRef]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Becquer, A.; Trap, J.; Irshad, U.; Ali, M.A.; Claude, P. From soil to plant, the journey of P through trophic relationships and ectomycorrhizal association. Front. Plant Sci. 2014, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Liang, G.; Yang, S.; Chen, C.; Huang, J. Influences of Laccaria bicolor on the growth, nutrient uptake and aluminum resistance of Pinus massoniana seedlings. Sci. Silvae Sin. 2005, 41, 199–203. [Google Scholar]
- Gu, X.; Huang, J. Effect of aluminum on growth, oxalate exudation, and uptake of aluminum, phosphorus and potassium by ectomycorrhizal fungi in vitro. Acta Ecol. Sin. 2010, 30, 0357–0363. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; Chinese Agricultural Press: Beijing, China, 2000; pp. 74–165. [Google Scholar]
- Shinde, S.; Naik, D.; Cumming, J.R. Carbon allocation and partitioning in Populus tremuloides are modulated by ectomycorrhizal fungi under phosphorus limitation. Tree Physiol. 2018, 38, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, L.; Tai, P.; Liu, W.; Li, X.; Hao, L. Effects of grafting on root-to-shoot cadmium translocation in plants of eggplant (Solanum melongena) and tomato (Solanum lycopersicum). Sci. Total Environ. 2019, 652, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.W.; Medve, R.J. Effects of aluminum and manganese on the growth of ectomycorrhizal fungi. Appl. Environ. Microbiol. 1984, 48, 556–560. [Google Scholar]
- Bucking, H.; Beckmann, S.; Heyser, W.; Kottke, I. Elemental contents in vacuolar granules of ectomycorrhizal fungi measured by EELS and EDXS. A comparison of different methods and preparation techniques. Micron 1998, 29, 53–61. [Google Scholar] [CrossRef]
- Costa, L.S.; Grazziotti, P.H.; Grazziotti, D.C.F.S.; Silva, A.C.; Rossi, M.J.; Silva, E.B.; Costa, V.H.D.; Gomes, A.L.F. In vitro evaluation of Eucalyptus ectomycorrhizae on substrate with phosphorus doses for fungal pre-selection. Rev. Árvore 2015, 39, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Schier, G.A.; McQuattie, C.J. Effect of aluminum on the growth, anatomy, and nutrient content of ectomycorrhizal and nonmycorrhizal eastern white pine seedlings. Can. J. For. Res. 1995, 25, 1252–1262. [Google Scholar] [CrossRef]
- Huang, J.; Lapeyrie, F. Ability of ectomycorrhizal fungus Laccaria bicolor S238N to increase the growth of Douglas fir seedlings and their phosphorus and potassium uptake. Pedosphere 1996, 6, 217–224. [Google Scholar]
- Choi, D.S.; Jin, H.O.; Chung, D.J.; Sasa, K.; Koike, T. Growth and physiological activity in Larix kaempferi seedlings inoculated with ectomycorrhizae as affected by soil acidification. Trees 2008, 22, 729–735. [Google Scholar] [CrossRef]
- Makita, N.; Hirano, Y.; Yamanaka, T.; Yoshimura, K.; Kosugi, Y. Ectomycorrhizal-fungal colonization induces physio-morphological changes in Quercus serrata leaves and roots. J. Plant Nutr. Soil Sci. 2012, 175, 900–906. [Google Scholar] [CrossRef]
- Fransson, P.M.A.; Anderson, I.C.; Alexander, I.J. Does carbon partitioning in ectomycorrhizal pine seedlings under elevated CO2 vary with fungal species? Plant Soil 2007, 291, 323–333. [Google Scholar] [CrossRef]
- Cairney, J.W.G. Extramatrical mycelia of ectomycorrhizal fungi as moderators of carbon dynamics in forest soil. Soil. Biol. Biochem. 2012, 47, 198–208. [Google Scholar] [CrossRef]
- Felten, J.; Martin, F.; Legué, V. Signaling in ectomycorrhizal symbiosis. In Signaling and Communication in Plants; Baluška, F., Vivanco, J., Eds.; Springer: Berlin, Germany, 2012; pp. 123–142. [Google Scholar]
- Szuba, A. Ectomycorrhiza of Populus. For. Ecol. Manag. 2015, 347, 156–169. [Google Scholar] [CrossRef]
- Szuba, A.; Karlinski, L.; Krzeslowska, M.; Hazubska-Przybyl, T. Inoculation with a Pb-tolerant strain of Paxillus involutus improves growth and Pb tolerance of Populus × canescens under in vitro conditions. Plant Soil 2017, 412, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Verlinden, M.S.; Verbruggen, E.; Janssens, I.A.; Wallander, H.; Vicca, S. Favorable effect of mycorrhizae on biomass production efficiency exceeds their carbon cost in a fertilization experiment. Ecology 2018, 99, 2525–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairney, J.W.G. Ectomycorrhizal fungi: The symbiotic route to the root for phosphorus in forest soils. Plant Soil 2011, 344, 51–71. [Google Scholar] [CrossRef]
- Aggangan, N.S.; Moon, H.K.; Han, S.H. Growth response of Acacia mangium Willd. seedlings to arbuscular mycorrhizal fungi and four isolates of the ectomycorrhizal fungus Pisolithus tinctorius (Pers.) Coker and Couch. New For. 2010, 39, 215–230. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Hoff, C.J.; Bryce, J.G.; Colpaert, J.V.; Hallett, R.A. Nutrient supply rate and mycorrhizal colonization control patterns of element distribution in ectomycorrhizal pine. Commun. Soil Sci. Plant Anal. 2009, 40, 3503–3523. [Google Scholar] [CrossRef]
- Smits, M.M.; Bonneville, S.; Benning, L.G.; Banwart, A.; Leake, J.R. Plant-driven weathering of apatite-the role of an ectomycorrhizal fungus. Geobiology 2012, 10, 445–456. [Google Scholar] [CrossRef]
- Gu, X.; Ni, Y.; Jiang, Y.; Jia, H.; He, X. Effect of Laccaria bicolor inoculation on contents of inorganic phosphorus and labile aluminum in the rhizosphere soil of Pinus massoniana saplings. Acta Pedol. Sin. 2018, 55, 1179–1189. [Google Scholar]
- Browning, M.H.R.; Hutchinson, T.C. The effects of aluminum and calcium on the growth and nutrition of selected ectomycorrhizal fungi of jack pine. Can. J. Bot. 1991, 69, 1691–1699. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Q.; Gu, X.; Wan, Y.; Mao, W.; Song, Y.; Zeng, Q. Resistance and nutritional response to aluminum toxicity of three ectomycorrhizal fungi from forest soils in Southwest China. J. Southwest Univ. Nat. Sci. Ed. 2015, 37, 13–19. [Google Scholar]
- Heim, A.; Brunner, I.; Frossard, E.; Luster, J. Aluminum effects on Picea abies at low solution concentrations. Soil Sci. Soc. Am. J. 2003, 67, 895–898. [Google Scholar] [CrossRef]
- Kayama, M.; Qu, L.; Koike, T. Elements and ectomycorrhizal symbiosis affecting the growth of Japanese larch seedlings regenerated on slopes of an active volcano in northern Japan. Trees Struct. Funct. 2015, 29, 1567–1579. [Google Scholar] [CrossRef]
- Fernández-Fuego, D.; Keunen, E.; Cuypers, A.; Bertrand, A.; González, A. Mycorrhization protects Betula pubescens Ehr. from metal-induced oxidative stress increasing its tolerance to grow in an industrial polluted soil. J. Hazard. Mater. 2017, 336, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shi, L.; Zhong, K.; Shen, Z.; Chen, Y. Ectomycorrhizal fungi may not act as a barrier inhibiting host plant absorption of heavy metals. Chemosphere 2019, 215, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.F.V.; Páez, F.A.R.; Vargas, S.E. Effect of arbuscular mycorrhizae and mercury on Lactuca sativa (Asteraceae) seedling morpho—Histology. Environ. Exp. Bot. 2018, 156, 197–202. [Google Scholar] [CrossRef]
- Phanthavongsa, P.; Chalot, M.; Papin, A.; Lacercat-Didier, L.; Roy, S.; Blaudez, D.; Bert, V. Effect of mycorrhizal inoculation on metal accumulation by poplar leaves at phytomanaged sites. Environ. Exp. Bot. 2017, 143, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Fuego, D.; Bertrand, A.; González, A. Metal accumulation and detoxification mechanisms in mycorrhizal Betula pubescens. Environ. Pollut. 2017, 231, 1153–1162. [Google Scholar] [CrossRef]
- Moyer-Henry, K.; Macfall, J.; Johannes, E.; Allen, N.; Goldfarb, B.; Rufty, T. Accumulation and localization of aluminum in root tips of loblolly pine seedlings and the associated ectomycorrhizal Pisolithus tinctorius. Plant Cell Environ. 2005, 28, 111–120. [Google Scholar] [CrossRef]
- Vaario, L.; Pennanen, T.; Lu, J.; Palmen, J.; Stenman, J.; Leveinen, J.; Kilpelainen, P.; Kitunen, V. Tricholoma matsutake can absorb and accumulate trace elements directly from rock fragments in the shiro. Micorrhiza 2015, 25, 325–334. [Google Scholar] [CrossRef]
- Wen, Z.; Shi, L.; Tang, Y.; Shen, Z.; Xia, Y.; Chen, Y. Effects of Pisolithus tinctorius and Cenococcum geophilum inoculation on pine in copper-contaminated soil to enhance phytoremediation. Int. J. Phytoremediation 2017, 19, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ni, Y.; Jiang, Y.; Jia, H. Effects of Laccaria bicolor on growth, uptake and distribution of nutrients and aluminum of Pinus massoniana seedlings under acid aluminum exposure. Sci. Silvae Sin. 2018, 54, 170–178. [Google Scholar]
- Liu, R.; Liu, H. Effect of acidity and aluminum on the growth of Pinus massoniana seedlings. Acta Bot. Sin. 1995, 37, 154–158. [Google Scholar]




| Variable | Source | Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|---|
| Biomass production | ECM inoculation | 95480.89 | 3 | 31826.96 | 129.49 *** | 0.000 |
| Al treatment | 194.37 | 1 | 194.37 | 0.791 ns | 0.387 | |
| ECM inoculation × Al treatment | 30019.84 | 3 | 10006.62 | 40.71 *** | 0.000 | |
| P accumulation | ECM inoculation | 0.12 | 3 | 0.04 | 130.80 *** | 0.000 |
| Al treatment | 0.05 | 1 | 0.05 | 149.42 *** | 0.000 | |
| ECM inoculation × Al treatment | 0.04 | 3 | 0.01 | 42.78 *** | 0.000 | |
| Ca accumulation | ECM inoculation | 2.15 | 3 | 0.72 | 70.69 *** | 0.000 |
| Al treatment | 0.00 | 1 | 0.00 | 0.11 ns | 0.750 | |
| ECM inoculation × Al treatment | 0.49 | 3 | 0.16 | 16.00 *** | 0.000 | |
| Mg accumulation | ECM inoculation | 0.20 | 3 | 0.07 | 209.85 *** | 0.000 |
| Al treatment | 0.01 | 1 | 0.01 | 26.37 *** | 0.000 | |
| ECM inoculation × Al treatment | 0.07 | 3 | 0.02 | 76.22 *** | 0.000 | |
| Shoot/root ratio | ECM inoculation | 6.85 | 3 | 2.28 | 5.36 ** | 0.010 |
| Al treatment | 2.43 | 1 | 2.43 | 5.71 * | 0.030 | |
| ECM inoculation × Al treatment | 22.29 | 3 | 7.43 | 17.45 *** | 0.000 | |
| P translocation factor | ECM inoculation | 92.22 | 3 | 30.74 | 107.04 *** | 0.000 |
| Al treatment | 7.98 | 1 | 7.98 | 27.77 *** | 0.000 | |
| ECM inoculation × Al treatment | 27.32 | 3 | 9.11 | 37.71 *** | 0.000 | |
| Ca translocation factor | ECM inoculation | 21.57 | 3 | 7.19 | 7.50 ** | 0.007 |
| Al treatment | 35.68 | 1 | 35.68 | 28.30 *** | 0.000 | |
| ECM inoculation × Al treatment | 34.60 | 3 | 11.53 | 9.15 *** | 0.001 | |
| Mg translocation factor | ECM inoculation | 278.60 | 3 | 92.87 | 16.49 *** | 0.000 |
| Al treatment | 356.25 | 1 | 356.25 | 63.25 *** | 0.000 | |
| ECM inoculation × Al treatment | 350.64 | 3 | 116.88 | 20.75 *** | 0.000 | |
| Al translocation factor | ECM inoculation | 0.46 | 3 | 0.15 | 10.98 *** | 0.000 |
| Al treatment | 1.19 | 1 | 1.19 | 84.42 *** | 0.000 | |
| ECM inoculation × Al treatment | 1.30 | 3 | 0.43 | 30.76 *** | 0.000 |
| Al Treatment | Model | B | β | t (7) | p | R2 | F | p |
|---|---|---|---|---|---|---|---|---|
| −Al | (Constant) | 65.03 | 3.40 * | 0.011 | 0.99 | 176.51 *** | 0.000 | |
| P accumulation | 228.28 | 0.27 | 2.07 ns | 0.078 | ||||
| Ca accumulation | 37.89 | 0.17 | 1.34 ns | 0.223 | ||||
| Mg accumulation | 271.84 | 0.38 | 1.77 ns | 0.120 | ||||
| Al accumulation | 158.87 | 0.19 | 0.99 ns | 0.356 | ||||
| +Al | (Constant) | −5.60 | −0.39 ns | 0.709 | 0.99 | 714.86 *** | 0.000 | |
| P accumulation | 234.74 | 0.24 | 4.46 ** | 0.003 | ||||
| Ca accumulation | 35.31 | 0.17 | 3.58 ** | 0.009 | ||||
| Mg accumulation | 399.33 | 0.61 | 7.54 *** | 0.000 | ||||
| Al accumulation | 89.52 | 0.05 | 2.32 ns | 0.054 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Wang, X.; Li, J.; He, X. Accumulation and Translocation of Phosphorus, Calcium, Magnesium, and Aluminum in Pinus massoniana Lamb. Seedlings Inoculated with Laccaria bicolor Growing in an Acidic Yellow Soil. Forests 2019, 10, 1153. https://doi.org/10.3390/f10121153
Gu X, Wang X, Li J, He X. Accumulation and Translocation of Phosphorus, Calcium, Magnesium, and Aluminum in Pinus massoniana Lamb. Seedlings Inoculated with Laccaria bicolor Growing in an Acidic Yellow Soil. Forests. 2019; 10(12):1153. https://doi.org/10.3390/f10121153
Chicago/Turabian StyleGu, Xirong, Xiaohe Wang, Jie Li, and Xinhua He. 2019. "Accumulation and Translocation of Phosphorus, Calcium, Magnesium, and Aluminum in Pinus massoniana Lamb. Seedlings Inoculated with Laccaria bicolor Growing in an Acidic Yellow Soil" Forests 10, no. 12: 1153. https://doi.org/10.3390/f10121153
APA StyleGu, X., Wang, X., Li, J., & He, X. (2019). Accumulation and Translocation of Phosphorus, Calcium, Magnesium, and Aluminum in Pinus massoniana Lamb. Seedlings Inoculated with Laccaria bicolor Growing in an Acidic Yellow Soil. Forests, 10(12), 1153. https://doi.org/10.3390/f10121153