The Effect of Forest Thinning on Soil Microbial Community Structure and Function
Abstract
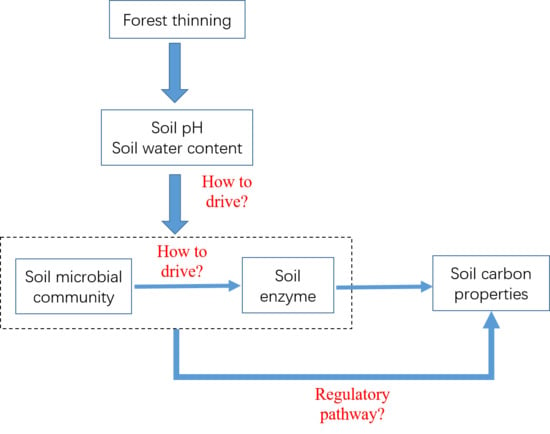
:1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Experimental Design and Treatments
Soil Property Analyses
2.3. Soil Microbial Community Structure and Function
2.4. Statistics
3. Results
3.1. Soil Parameters
3.2. Soil Microbial Community Structure
3.3. Soil Enzyme Activities
3.4. Correlation between Soil Microbial Community Structure and Soil Enzyme Activities
3.5. Effects of Soil PH and Soil Water Content on Soil Microbial Community
3.6. Effects of Soil PH, Soil Water Content, and Microbial Factors on Soil Carbon Properties
4. Discussion
4.1. Soil Microbial Community Structure and Soil Enzyme in Thinning Treatments
4.2. Correlation between Soil Microbial Community Structure and Soil Enzyme Activities, Controlled by Soil PH and Soil Water Content
4.3. Effects of Soil PH, Soil Water Content, and Microbial Factors on Soil Carbon Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gömöryová, E.; Hrivnák, R.; Janišová, M.; Ujházy, K.; Gömöry, D. Changes of the functional diversity of soil microbial community during the colonization of abandoned grassland by a forest. Appl. Soil Ecol. 2009, 43, 191–199. [Google Scholar] [CrossRef]
- Paulinem, M.; Davide, C. Application of self-organizing maps for assessing soil biological quality. Agric. Ecosyst. Environ. 2008, 126, 139–152. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Zhang, L.; Han, X.H.; Yang, G.H.; Wang, J. Changes in soil microbial community are linked to soil carbon fractions after afforestation. Eur. J. Soil Sci. 2018. [Google Scholar] [CrossRef]
- Lei, L.; Xiao, W.; Zeng, L.; Zhu, J.; Huang, Z.; Cheng, R.; Gao, S.; Li, M. Thinning but not understory removal increased heterotrophic respiration and total soil respiration in Pinus massoniana stands. Sci. Total Environ. 2018, 621, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Schoning, I.; Kahl, T.; Bauhus, J.; Ruess, L. Regional environmental conditions shape microbial community structure stronger than local forest management intensity. For. Ecol. Manag. 2018, 409, 250–259. [Google Scholar] [CrossRef]
- Yang, X.Q.; Han, Y.Z.; Le, L.I.; Xin, C. The effect of heterogeneous spatial distribution of soil nitrogen on regeneration of Larix principis-rupprechtii seedlings in typical naturally-regenerated montane forests of Northen China. Acta Ecol. Sin. 2009, 29, 4656–4664. [Google Scholar] [CrossRef]
- Saunders, M.; Tobin, B.; Black, K.; Gioria, M.; Nieuwenhuis, M.; Osborne, B.A. Thinning effects on the net ecosystem carbon exchange of a Sitka spruce forest are temperature-dependent. Agric. For. Meteorol. 2012, 157, 1–10. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Z.; Zha, T.; Luo, Z.; Zheng, J.; Sun, O.J. Predicting soil respiration using carbon stock in roots, litter and soil organic matter in forests of Loess Plateau in China. Soil Biol. Biochem. 2013, 57, 135–143. [Google Scholar] [CrossRef]
- Bennett, J.A.; Maherali, H.; Reinhart, K.O.; Lekberg, Y.; Hart, M.M.; Klironomos, J. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef]
- Yang, B.; Pang, X.; Hu, B.; Bao, W.; Tian, G. Does thinning-induced gap size result in altered soil microbial community in pine plantation in eastern Tibetan Plateau? Ecol. Evol. 2017, 7, 2986–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, D.; Chen, X.; Wang, J.; Diao, J.; Zhang, J.; Guan, Q. Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl. Soil Ecol. 2015, 92, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Bowman, D.; Shi, W. Soil microbial community structure and diversity in a turfgrass chronosequence: Land-use change versus turfgrass management. Appl. Soil Ecol. 2006, 34, 0–218. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, N.; Ge, T.; Kuzyakov, Y.; Wang, Z.L.; Li, Z.; Tang, Z.; Chen, Y.; Wu, C.; Lou, Y. Soil aggregation regulates distributions of carbon, microbial community and enzyme activities after 23-year manure amendment. Appl. Soil Ecol. 2017, 111, 65–72. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Dong, B.; Zhou, M.; Ma, L.; Jia, Z.; Duan, J. Regeneration response to canopy gap size in a Chinese pine plantation: Species diversity patterns, size structures and spatial distributions. For. Ecol. Manag. 2017, 397, 97–107. [Google Scholar] [CrossRef]
- Wang, R.; Dorodnikov, M.; Yang, S.; Zhang, Y.; Filley, T.R.; Turco, R.F.; Zhang, Y.; Xu, Z.; Li, H.; Jiang, Y. Responses of enzymatic activities within soil aggregates to 9-year nitrogen and water addition in a semi-arid grassland. Soil Biol. Biochem. 2015, 81, 159–167. [Google Scholar] [CrossRef]
- Li, J.; Cooper, J.M.; Lin, Z.; Li, Y.; Yang, X.; Zhao, B. Soil microbial community structure and function are significantly affected by long-term organic and mineral fertilization regimes in the North China Plain. Appl. Soil Ecol. 2015, 96, 75–87. [Google Scholar] [CrossRef]
- Claassens, S.; van Rensburg, P.J.; Maboeta, M.S.; van Rensburg, L. Soil microbial community function and structure in a post-mining Chronosequence. Water Air Soil Pollut. 2008, 194, 315–329. [Google Scholar] [CrossRef]
- Brant, J.B.; Sulzman, E.W.; Myrold, D.D. Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol. Biochem. 2006, 38, 2219–2232. [Google Scholar] [CrossRef]
- Sylvia, D.M.; Fuhrmann, J.J.; Hartel, P.; Zuberer, D.A. Principles and applications of soil microbiology. New Age Int. 1998, 34, 11–68. [Google Scholar]
- Ma, J.; Kang, F.; Cheng, X.; Han, H. Moderate thinning increases soil organic carbon in Larix principis-rupprechtii (pinaceae) plantations. Geoderma 2018, 329, 118–128. [Google Scholar] [CrossRef]
- Cheng, X.; Han, H.; Kang, F.; Liu, K.; Song, Y.; Zhou, B.; Li, Y. Short-term effects of thinning on soil respiration in a pine (Pinus tabulaeformis) plantation. Biol. Fert. Soils 2014, 50, 357–367. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, M.; Li, Z. Short term effects of thinning on soil organic carbon fractions, soil properties, and forest floor in Cunninghamia lanceolata plantations. J. Soil Sci. Environ. Manag. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Clarke, N.; Gundersen, P.; Jönsson-Belyazid, U.; Kjønaas, O.J.; Persson, T.; Sigurdsson, B.D.; Stupak, I.; Vesterdal, L. Influence of different tree-harvesting intensities on forest soil carbon stocks in boreal and northern temperate forest ecosystems. For. Ecol. Manag. 2015, 351, 9–19. [Google Scholar] [CrossRef]
- Georgiou, K.; Abramoff, R.Z.; Harte, J.; Riley, W.J.; Torn, M.S. Microbial community-level regulation explains soil carbon responses to long-term litter manipulations. Nat. Commun. 2018, 9, 1223. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, W.; Yu, H.; He, X. Linking organic carbon accumulation to microbial community dynamics in a sandy loam soil: Result of 20 years compost and inorganic fertilizers repeated application experiment. Biol. Fertil. Soils 2015, 51, 137–150. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Dannenmann, M.; Gasche, R.; Ledebuhr, A.; Papen, H. Effects of forest management on soil N cycling in beech forests stocking on calcareous soils. Plant Soil 2006, 287, 279–300. [Google Scholar] [CrossRef]
- Sauze, J.; Jones, S.P.; Wingate, L.; Wohl, S.; Ogée, J. The role of soil pH on soil carbonic anhydrase activity. Biogeosci. Discuss 2018, 15, 1–28. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, R.; Shen, Y.; Marschner, P. Soil water content during and after plant growth influence nutrient availability and microbial biomass. J. Soil Sci. Plant Nutr. 2017, 17, 702–715. [Google Scholar] [CrossRef] [Green Version]
- Drenovsky, R.E.; Vo, D.; Graham, K.J.; Scow, K.M. Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef]
- You, Y.; Wang, J.; Huang, X.; Tang, Z.; Liu, S.; Sun, O.J. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol. Evol. 2014, 4, 633–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grayston, S.J.; Prescott, C.E. Microbial communities in forest floors under four tree species in coastal British Columbia. Soil Biol. Biochem. 2005, 37, 1157–1167. [Google Scholar] [CrossRef]
- Yao, H.; Chapman, S.J.; Thornton, B.; Paterson, E. 13 C PLFAs: A key to open the soil microbial black box? Plant Soil 2015, 392, 3–15. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Overby, S.T.; Owen, S.M.; Hart, S.C.; Neary, D.G.; Johnson, N.C. Soil microbial community resilience with tree thinning in a 40-year-old experimental ponderosa pine forest. Appl. Soil Ecol. 2015, 93, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, Y.; Zhou, H.; Zhao, G.; Wang, L. Effects of gaps in the forest canopy on soil microbial communities and enzyme activity in a Chinese pine forest. Pedobiologia 2017, 61, 51–60. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Yao, Q.; Yu, S.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Diversity and distribution patterns of acidobacterial communities in the black soil zone of northeast China. Soil Biol. Biochem. 2016, 95, 212–222. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Y.; Fu, X.; Xu, M.; Dai, X.; Wang, H. Thinning effect on understory community and photosynthetic characteristics in a subtropical Pinus massoniana plantation. Can. J. For. Res. 2017, 47, 1104–1115. [Google Scholar] [CrossRef]
- Trentini, C.P.; Campanello, P.I.; Villagra, M.; Ritter, L.; Ares, A.; Goldstein, G. Thinning of loblolly pine plantations in subtropical Argentina: Impact on microclimate and understory vegetation. For. Ecol. Manag. 2017, 384, 236–247. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H.; Chen, X.; Wang, J.; Chen, B.; Wang, D.; Guan, Q. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations. Appl. Soil Ecol. 2016, 107, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Li, G.; Han, S.H.; Kim, H.J.; Kim, C.; Lee, S.T.; Son, Y. Thinning affects microbial biomass without changing enzyme activity in the soil of Pinus densiflora Sieb. et Zucc. forests after 7 years. Ann. For. Sci. 2018, 75, 13. [Google Scholar] [CrossRef]
- Rasche, F.; Knapp, D.; Kaiser, C.; Koranda, M.; Kitzler, B.; Zechmeister-Boltenstern, S.; Richter, A.; Sessitsch, A. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J. 2011, 5, 389–402. [Google Scholar] [CrossRef]
- Urbanová, M.; Anajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Hester, A.J.; Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Hewison, R.L.; Potts, J.M. Is vegetation composition or soil chemistry the best predictor of the soil microbial community? Plant Soil 2010, 333, 417–430. [Google Scholar] [CrossRef]
- Rietl, A.J.; Jackson, C.R. Effects of the ecological restoration practices of prescribed burning and mechanical thinning on soil microbial enzyme activities and leaf litter decomposition. Soil Biol. Biochem. 2012, 50, 47–57. [Google Scholar] [CrossRef]
- Bell, T.H.; Klironomos, J.N.; Henry, H.A.L. Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen addition. Soil Sci. Soc. Am. J. 2010, 74, 820–828. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Firestone, M.K. Altered utilization patterns of young and old soil C by microorganisms caused by temperature shifts and N additions. Biogeochemistry 2004, 67, 235–248. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef]
- Ren, C.; Wang, T.; Xu, Y.; Deng, J.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. For. Ecol. Manag. 2018, 409, 170–178. [Google Scholar] [CrossRef]
- Tardy, V.; Spor, E.; Mathieu, O.; Eque, J.L.E.; Terrat, S.E.; Plassart, P.; Regnier, T.; Bardgett, R.D.; Putten, W.H.V.D.; Roggero, P.P. Shifts in microbial diversity through land use intensity as drivers of carbon mineralization in soil. Soil Biol. Biochem. 2015, 90, 204–213. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, H.; Xing, F.; Bai, Z.; Gao, Y.; Dong, Y.; Zhou, J.; Wu, Y.; Yang, Y. Response of soil microbial community structure to increased precipitation and nitrogen addition in a semiarid meadow steppe. Eur. J. Soil. Sci. 2017, 68, 524–536. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Banerjee, S.; Zhou, N.; Zhao, Z.; Zhang, K.; Tian, C. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Baah Acheamfour, M.; Carlyle, C.N.; Bissett, A.; Richardson, A.E.; Siddique, T.; Bork, E.W.; Chang, S.X. Determinants of bacterial communities in Canadian agroforestry systems. Environ. Microbiol. 2016, 18, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Chen, H.Y.H. Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: Literature review and meta-analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Budge, K.; Leifeld, J.; Hiltbrunner, E.; Fuhrer, J. Litter quality and pH are strong drivers of carbon turnover and distribution in alpine grassland soils. Biogeosci. Discuss. 2010, 7, 6207–6242. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H.; Chen, Q.; Han, X. The counteractive effects of nitrogen addition and watering on soil bacterial communities in a steppe ecosystem. Soil Biol. Biochem. 2014, 72, 26–34. [Google Scholar] [CrossRef]
- St-Arnaud, M.; Hamel, C.; Vimard, B.; Caron, M.; Fortin, J.A. Altered growth of Fusarium oxysporum f. sp. chrysanthemi in an in vitro dual culture system with the vesicular arbuscular mycorrhizal fungus Glomus intraradices growing on Daucus carota transformed roots. Mycorrhiza 1995, 5, 431–438. [Google Scholar] [CrossRef]




| Months | Treatments | Shannon–Wiener index | Species Richness | Species Evenness |
|---|---|---|---|---|
| August, 2015 | CK | 3.38 ± 0.01b | 78.50 ± 0.71a | 0.78 ± 0.00a |
| LT | 3.40 ± 0.01b | 78.33 ± 1.15a | 0.78 ± 0.01a | |
| MT | 3.47 ± 0.03a | 79.00 ± 5.57a | 0.79 ± 0.02a | |
| HT | 3.42 ± 0.02b | 80.00 ± 2.65a | 0.78 ± 0.01a | |
| April, 2016 | CK | 3.22 ± 0.00a | 54.00 ± 2.83a | 0.81 ± 0.01a |
| LT | 3.12 ± 0.07a | 50.00 ± 2.65a | 0.80 ± 0.01a | |
| MT | 3.14 ± 0.09a | 52.00 ± 2.65a | 0.79 ± 0.01a | |
| HT | 3.21 ± 0.01a | 54.33 ± 0.58a | 0.80 ± 0.00a | |
| June, 2016 | CK | 3.27 ± 0.08b | 55.00 ± 4.24c | 0.82 ± 0.01a |
| LT | 3.29 ± 0.06b | 54.67 ± 1.53c | 0.82 ± 0.01a | |
| MT | 3.38 ± 0.03ab | 62.67 ± 3.21b | 0.82 ± 0.00a | |
| HT | 3.43 ± 0.02a | 69.33 ± 2.31a | 0.81 ± 0.00a | |
| August, 2016 | CK | 3.40 ± 0.02a | 60.50 ± 0.71a | 0.83 ± 0.01a |
| LT | 3.41 ± 0.12a | 64.33 ± 7.37a | 0.82 ± 0.01a | |
| MT | 3.37 ± 0.12a | 65.67 ± 7.09a | 0.81 ± 0.01a | |
| HT | 3.33 ± 0.04a | 59.33 ± 6.03a | 0.82 ± 0.03a | |
| October, 2016 | CK | 3.46 ± 0.01a | 70.50 ± 3.54ab | 0.81 ± 0.01a |
| LT | 3.39 ± 0.04b | 63.67 ± 2.52b | 0.82 ± 0.01a | |
| MT | 3.40 ± 0.04b | 68.00 ± 9.00ab | 0.81 ± 0.02a | |
| HT | 3.47 ± 0.00a | 78.00 ± 2.00a | 0.80 ± 0.00a |
| Microbial Composition | Time of Sampling | Repeated Measurement | ||||
|---|---|---|---|---|---|---|
| August, 2015 | April, 2016 | June, 2016 | August, 2016 | October, 2016 | ||
| Total PLFAs | 1.63 (0.27) | 2.36 (0.16) | 63.83 (0.00) *** | 1.19 (0.38) | 11.19 (0.01) ** | 116.27 (0.00) *** |
| Gram-positive bacteria | 1.77 (0.24) | 1.70 (0.25) | 36.60 (0.00) *** | 1.00 (0.45) | 9.72 (0.01) ** | 302.55 (0.00) *** |
| Gram-negative bacteria | 0.72 (0.57) | 0.60 (0.64) | 45.70 (0.00) *** | 0.93 (0.48) | 8.57 (0.01) ** | 142.09 (0.00) *** |
| AMF | 1.08 (0.42) | 1.27 (0.38) | 74.39 (0.00) *** | 0.70 (0.58) | 8.77 (0.01) ** | 91.20 (0.00) *** |
| Sap | 1.07 (0.42) | 0.682 (0.59) | 2.16 (0.18) | 0.67 (0.60) | 2.08 (0.19) | 17.96 (0.01) ** |
| Actinomycetes | 0.85 (0.51) | 0.94 (0.47) | 97.27 (0.00) *** | 0.75 (0.56) | 11.94 (0.00) ** | 242.71 (0.00) *** |
| Microbial Composition | Thinning | Time of Sampling | ||||
|---|---|---|---|---|---|---|
| August, 2015 | April, 2016 | June, 2016 | August, 2016 | October, 2016 | ||
| Total PLFAs | CK | 83.21 ± 1.82a | 16.21 ± 1.72ab | 17.08 ± 1.26a | 19.23 ± 3.34a | 34.29 ± 3.33a |
| LT | 81.84 ± 7.41a | 15.00 ± 1.34a | 18.57 ± 1.31a | 23.96 ± 5.73a | 24.15 ± 2.81b | |
| MT | 91.23 ± 12.81a | 15.79 ± 2.65ab | 27.64 ± 1.97b | 25.97 ± 6.17a | 28.03 ± 5.16b | |
| HT | 75.46 ± 7.12a | 18.91 ± 1.59b | 36.05 ± 2.28c | 19.58 ± 3.34a | 40.98 ± 3.22c | |
| Gram-positive bacteria | CK | 22.02 ± 0.49a | 3.00 ± 0.50a | 3.41 ± 0.39a | 4.41 ± 1.56a | 8.31 ± 0.10a |
| LT | 20.99 ± 1.96a | 2.85 ± 0.46a | 3.81 ± 0.35a | 4.91 ± 1.61a | 5.50 ± 0.79b | |
| MT | 24.45 ± 4.16a | 2.86 ± 0.76a | 6.31 ± 0.65b | 6.06 ± 1.64a | 6.59 ± 1.59b | |
| HT | 19.51 ± 2.05a | 3.71 ± 0.33a | 8.80 ± 1.00c | 4.15 ± 0.81a | 10.58 ± 1.27c | |
| Gram-negative bacteria | CK | 23.27 ± 1.70a | 2.94 ± 0.81a | 3.11 ± 0.36a | 3.20 ± 0.99a | 7.71 ± 1.07a |
| LT | 23.57 ± 2.57a | 2.75 ± 0.47a | 3.54 ± 0.49a | 3.94 ± 1.45a | 4.69 ± 1.25b | |
| MT | 25.00 ± 5.71a | 2.70 ± 0.86a | 5.49 ± 0.61b | 4.81 ± 1.60a | 6.27 ± 1.27b | |
| HT | 20.77 ± 2.12a | 3.33 ± 0.39a | 8.02 ± 0.61c | 3.32 ± 0.72a | 9.03 ± 0.66c | |
| AMF | CK | 2.10 ± 0.11a | 0.26 ± 0.04a | 0.28 ± 0.04a | 0.28 ± 0.09a | 0.76 ± 0.14ab |
| LT | 2.39 ± 0.40a | 0.25 ± 0.05a | 0.31 ± 0.01a | 0.36 ± 0.14a | 0.50 ± 0.08a | |
| MT | 2.78 ± 0.73a | 0.27 ± 0.09a | 0.56 ± 0.06b | 0.44 ± 0.17a | 0.70 ± 0.19a | |
| HT | 2.23 ± 0.20a | 0.34 ± 0.03a | 0.76 ± 0.05c | 0.31 ± 0.10a | 1.02 ± 0.07b | |
| Sap | CK | 1.37 ± 0.18a | 0.17 ± 0.06a | 0.17 ± 0.01a | 0.39 ± 0.16a | 0.43 ± 0.06ab |
| LT | 1.26 ± 0.08a | 0.16 ± 0.06a | 0.26 ± 0.11ab | 0.37 ± 0.13a | 0.37 ± 0.12ab | |
| MT | 1.54 ± 0.83a | 0.12 ± 0.04a | 0.31 ± 0.10ab | 0.28 ± 0.04a | 0.31 ± 0.03a | |
| HT | 0.88 ± 0.18a | 0.17 ± 0.04a | 0.38 ± 0.10b | 0.30 ± 0.01a | 0.50 ± 0.13b | |
| Actinmycete | CK | 8.15 ± 0.58a | 1.08 ± 0.16a | 1.25 ± 0.16a | 1.42 ± 0.38a | 3.00 ± 0.23a |
| LT | 7.84 ± 0.43a | 1.01 ± 0.09a | 1.40 ± 0.12a | 1.59 ± 0.55a | 1.95 ± 0.28b | |
| MT | 7.89 ± 1.07a | 1.06 ± 0.28a | 2.31 ± 0.18b | 1.91 ± 0.47a | 2.33 ± 0.50b | |
| HT | 7.15 ± 0.74a | 1.24 ± 0.09a | 3.00 ± 0.07c | 1.42 ± 0.33a | 3.42 ±0.12a | |
| Soil Enzyme | Time of Sampling | Repeated Measurement | ||||
|---|---|---|---|---|---|---|
| August, 2015 | April, 2016 | June, 2016 | August, 2016 | October, 2016 | ||
| Phenol oxidase | 9.53 (0.01) ** | 21.98 (0.00) ** | 4.52 (0.05) * | 14.93 (0.00) ** | 6.17 (0.02) * | 96.82 (0.00) *** |
| Peroxidase | 3.42 (0.08) | 14.10 (0.00) ** | 6.05 (0.02) * | 0.94 (0.47) | 5.66 (0.03) * | 56.41 (0.00) *** |
| BG | 1.85 (0.23) | 2.23 (0.17) | 31.99 (0.00) *** | 5.01 (0.04) * | 0.69 (0.59) | 37.09 (0.00) ** |
| NAG | 7.99 (0.01) * | 4.81 (0.04) * | 34.76 (0.00) *** | 16.56 (0.00) ** | 2.58 (0.14) | 26.72 (0.00) *** |
| CBH | 7.23 (0.02) * | 6.89 (0.02) * | 71.10 (0.00) *** | 28.32 (0.00) *** | 23.78 (0.00) *** | 4.88 (0.00) ** |
| Variables | Lambda-A 1 | Lambda-B 2 | P3 | F-Ratio 4 |
|---|---|---|---|---|
| Actinomycetes | 0.18 | 0.18 | 0.01 | 4.03 |
| Gram-negative bacteria | 0.30 | 0.20 | 0.00 | 5.41 |
| Gram-negative bacteria | 0.13 | 0.02 | 0.61 | 0.66 |
| AMF | 0.26 | 0.01 | 0.92 | 0.20 |
| Variables | Lambda-A 1 | Lambda-B 2 | p3 | F-Ratio 4 |
|---|---|---|---|---|
| Soil pH | 0.53 | 0.53 | 0.00 | 42.83 |
| NAG | 0.23 | 0.08 | 0.00 | 10.37 |
| peroxidase | 0.19 | 0.06 | 0.00 | 9.23 |
| Shannon–Wiener index | 0.26 | 0.04 | 0.00 | 6.94 |
| Phenol oxidase | 0.25 | 0.03 | 0.02 | 4.81 |
| Soil water content | 0.22 | 0.00 | 0.79 | 0.22 |
| BG | 0.16 | 0.00 | 0.93 | 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Cheng, X.; Han, H. The Effect of Forest Thinning on Soil Microbial Community Structure and Function. Forests 2019, 10, 352. https://doi.org/10.3390/f10040352
Wu R, Cheng X, Han H. The Effect of Forest Thinning on Soil Microbial Community Structure and Function. Forests. 2019; 10(4):352. https://doi.org/10.3390/f10040352
Chicago/Turabian StyleWu, Ran, Xiaoqin Cheng, and Hairong Han. 2019. "The Effect of Forest Thinning on Soil Microbial Community Structure and Function" Forests 10, no. 4: 352. https://doi.org/10.3390/f10040352
APA StyleWu, R., Cheng, X., & Han, H. (2019). The Effect of Forest Thinning on Soil Microbial Community Structure and Function. Forests, 10(4), 352. https://doi.org/10.3390/f10040352