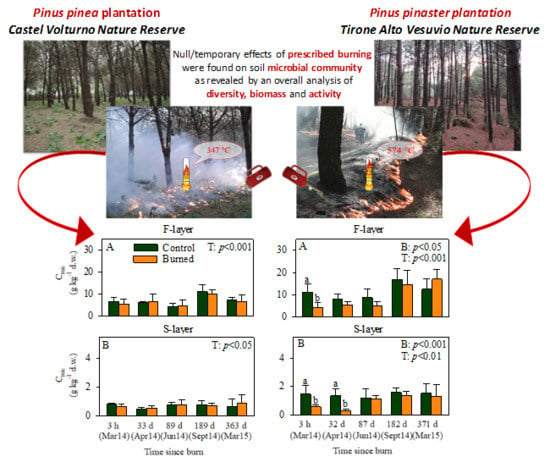
Soil Microbial Diversity, Biomass, and Activity in Two Pine Plantations of Southern Italy Treated with Prescribed Burning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Prescribed Burning Treatment
2.3. Sampling Protocol and Laboratory Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Effect of PB on Soil Microbial Community
4.2. Interrelationships among Variables
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adhikari, K.; Hartemink, A.E. Linking soils to ecosystem services—A global review. Geoderma 2016, 262, 101–111. [Google Scholar] [CrossRef]
- Saccá, M.L.; Barra Caracciolo, A.; Di Lenola, M.; Grenni, P. Ecosystem services provided by soil microorganisms. In Soil Biological Communities and Ecosystem Resilience; Lukac, M., Grenni, P., Gamboni, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 9–24. [Google Scholar]
- Singh, J.S.; Gupta, V.K. Soil microbial biomass: A key soil driver in management of ecosystem functioning. Sci. Total Environ. 2018, 634, 497–500. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Hawke, B.G.; McDonald, H.J.; Kirby, C.A.; Buckerfield, J.C.; Michelsen, P.; O’Brien, K.A.; Gupta, V.V.S.R.; Doube, B.M. Evaluation of soil biological properties as potential bioindicators of soil health. Aust. J. Exp. Agric. 1995, 35, 1015–1028. [Google Scholar] [CrossRef]
- Powlson, D.S.; Brookes, P.C.; Christensen, B.T. Measurement of soil microbial biomass provides an early indication of changes in the total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J. Plant Nutr. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Pulleman, M.M.; Creamer, R.; Hamer, U.; Helder, J.; Pelosi, C.; Pérès, G.; Rutgers, M. Soil biodiversity, biological indicators and soil ecosystem services—An overview of European approaches. Curr. Opin. Environ. Sustain. 2012, 4, 529–538. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Trabaud, L.; Grandjanny, M. Post-fire reconstitution of the flowering phenology in Mediterranean shrubland plants. In Fire and Biological Processes; Trabaud, L., Prodon, R., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2002; pp. 99–113. [Google Scholar]
- Turco, M.; von Hardenberg, J.; AghaKouchak, A.; Llasat, M.C.; Provenzale, A.; Trigo, R.M. On the key role of droughts in the dynamics of summer fires in Mediterranean Europe. Sci. Rep. 2017, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.; González-Pérez, J.A.; Turmero, A.; Hernández, M.; Ball, A.S.; González-Vila, F.J.; Arias, M.E. Wildfire effects on the microbial activity and diversity in a Mediterranean forest soil. Catena 2017, 158, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Fierro, A.; Rutigliano, F.A.; De Marco, A.; Castaldi, S.; Virzo De Santo, A. Post-fire stimulation of soil biogenic emission of CO2 in a sandy soil of a Mediterranean shrubland. Int. J. Wildland Fire 2007, 16, 573–583. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; De Marco, A.; D’Ascoli, R.; Castaldi, S.; Gentile, A.; Virzo De Santo, A. Impact of fire on fungal abundance and microbial efficiency in C assimilation and mineralisation in a Mediterranean maquis soil. Biol. Fertil. Soils 2007, 44, 377–381. [Google Scholar] [CrossRef]
- Fernandes, P.; Botelho, H. A review of prescribed burning effectiveness in fire hazard reduction. Int. J. Wildland Fire 2003, 12, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Furlaud, J.M.; Grant, A.B.J.; Williamson, A.; David, M.J.; Bowma, S. Simulating the effectiveness of prescribed burning at altering wildfire behaviour in Tasmania, Australia. Int. J. Wildland Fire 2017, 27, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.M.; Davies, G.M.; Ascoli, D.; Fernández, C.; Moreira, F.; Rigolot, E.; Stoof, C.R.; Vega, J.A.; Molina, D. Prescribed burning in southern Europe: Developing fire management in a dynamic landscape. Front. Ecol. Environ. 2013, e4–e14. [Google Scholar] [CrossRef] [Green Version]
- Piñol, J.; Beven, K.; Viegas, D. Modelling the effect of fire-exclusion and prescribed fire on wildfire size in Mediterranean ecosystems. Ecol. Model. 2005, 183, 397–409. [Google Scholar] [CrossRef]
- Cassagne, N.; Pimont, F.; Dupuy, J.L.; Linn, R.R.; Marell, A.; Oliveri, C.; Rigolot, E. Using a fire propagation model to assess the efficiency of prescribed burning in reducing the fire hazard. Ecol. Model. 2011, 222, 1502–1514. [Google Scholar] [CrossRef]
- Marino, E.; Guijarro, M.; Hernando, C.; Madrigal, J.; Díez, C. Fire hazard after prescribed burning in a gorse shrubland: Implications for fuel management. J. Environ. Manag. 2011, 92, 1003–1011. [Google Scholar] [CrossRef]
- Ascoli, D.; Catalanotti, A.E.; Valese, E.; Cabiddu, S.; Delogu, G.; Driussi, M.; Esposito, A.; Leone, V.; Lovreglio, R.; Marchi, E.; et al. Esperienze di fuoco prescritto in Italia: Un approccio integrato per la prevenzione degli incendi boschivi. Forest@ 2012, 9, 20–38. [Google Scholar] [CrossRef] [Green Version]
- Casals, P.; Valor, T.; Besalú, A.; Molina-Terrén, D. Under story fuel load and structure eight to nine years after prescribed burning in Mediterranean pine forests. Forest Ecol. Manag. 2016, 362, 156–168. [Google Scholar] [CrossRef]
- Espinosa, J.; Palheiro, P.; Loureiro, C.; Ascoli, D.; Esposito, A.; Fernandes, P.M. Fire-severity mitigation by prescribed burning assessed from fire-treatment encounters in maritime pine stands. Can. J. Forest Res. 2018, 49, 205–211. [Google Scholar] [CrossRef]
- Battipaglia, G.; Strumia, S.; Esposito, A.; Giuditta, E.; Sirignano, C.; Altieri, S.; Rutigliano, F.A. The effects of prescribed burning on Pinus halepensis Mill. as revealed by dendrochronological and isotopic analyses. Forest Ecol. Manag. 2014, 334, 201–208. [Google Scholar] [CrossRef]
- Battipaglia, G.; Savi, T.; Ascoli, D.; Castagneri, D.; Esposito, A.; Mayr, S.; Nardini, A. Effects of prescribed burning on ecophysiological, anatomical and stem hydraulic properties in Pinus pinea L. Tree Physiol. 2016, 36, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Battipaglia, G.; Tognetti, R.; Valese, E.; Ascoli, D.; De Luca, P.F.; Basile, S.; Ottaviano, M.; Mazzoleni, S.; Marchetti, M.; Esposito, A. Incendi 2017: Un’importante lezione. Forest@ 2017, 14, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.; Botelho, H.; Rego, F.C. A piroecologia do pinheiro bravo. Silva Lusit. 2005, 13, 233–248. [Google Scholar]
- Fan, Z.; Ma, Z.; Dey, D.C.; Roberts, S.D. Response of advance reproduction of oaks and associated species to repeated prescribed fires in upland oak-hickory forests, Missouri. Forest Ecol. Manag. 2012, 266, 160–169. [Google Scholar] [CrossRef]
- Fuentes, L.; Duguy, B.; Nadal-Sala, D. Short-term effects of spring prescribed burning on the understory vegetation of a Pinus halepensis forest in Northeastern Spain. Sci. Total Environ. 2018, 610–611, 720–731. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Urban, D.L.; White, P.S.; Moody, A.; Klein, R.N. Vegetation dynamics vary across topographic and fire severity gradients following prescribed burning in Great Smoky Mountains National Park. For. Ecol. Manag. 2016, 365, 1–11. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Ubeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613–614, 944–957. [Google Scholar] [CrossRef]
- Catalanotti, A.E.; Giuditta, E.; Marzaioli, R.; Ascoli, D.; Esposito, A.; Strumia, S.; Mazzoleni, S.; Rutigliano, F.A. Effects of single and repeated prescribed burns on soil organic C and microbial activity in a Pinus halepensis plantation of Southern Italy. Appl. Soil Ecol. 2017, 125, 108–116. [Google Scholar] [CrossRef]
- Shen, J.P.; Chen, C.R.; Lewis, T. Long term repeated fire disturbance alters soil bacterial diversity but not the abundance in an Australian wet sclerophyll forest. Sci. Rep. 2016, 6, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.J.; Hallgren, S.W.; Wilson, G.W.T. Frequency of prescribed burning in an upland oak forest determines soil and litter properties and alters the soil microbial community. For. Ecol. Manag. 2012, 265, 241–247. [Google Scholar] [CrossRef]
- Fritze, H.; Pennanen, T.; Pietikäinen, J. Recovery of soil microbial biomass and activity from prescribed burning. Can. J. For. Res. 1993, 23, 1286–1290. [Google Scholar] [CrossRef]
- Hamman, S.T.; Burke, I.C.; Knapp, E.E. Soil nutrients and microbial activity after early and late season prescribed burns in a Sierra Nevada mixed conifer forest. For. Ecol. Manag. 2008, 256, 367–374. [Google Scholar] [CrossRef]
- McCarthy, D.R.; Brown, K.J. Soil respiration responses to topography, canopy cover, and prescribed burning in an oak-hickory forest in southeastern Ohio. For. Ecol. Manag. 2006, 237, 94–102. [Google Scholar] [CrossRef]
- Fultz, L.M.; Moore-Kucera, J.; Dathe, J.; Davinic, M.; Perry, G.; Wester, D.; Schwilk, D.W.; Rideout-Hanzak, S. Forest wildfire and grassland prescribed fire effects on soil biogeochemical processes and microbial communities: Two case studies in the semi-arid Southwest. Appl. Soil Ecol. 2016, 99, 118–128. [Google Scholar] [CrossRef]
- Switzer, J.M.; Hope, G.D.; Grayston, S.J.; Prescott, C.E. Changes in soil chemical and biological properties after thinning and prescribed fire for ecosystem restoration in a Rocky Mountain Douglas-fir forest. For. Ecol. Manag. 2012, 275, 1–13. [Google Scholar] [CrossRef]
- Croce, A.; Stinca, A.; Santangelo, A.; Esposito, A. Exploring vascular flora biodiversity of two protected sandy coastal areas in southern Italy. Rend. Lincei 2019, 30, 323–336. [Google Scholar] [CrossRef]
- Esposito, A.; Mazzoleni, S.; Strumia, S. Post-fire bryophyte dynamics in Mediterranean vegetation. J. Veg. Sci. 1999, 10, 261–268. [Google Scholar] [CrossRef]
- di Gennaro, A. I Sistemi di Terre Della Campania. 2002. Available online: https://www.iris.unina.it/handle/11588/179891#.XfyHZmZ5uUk (accessed on 5 November 2019).
- Niccoli, F.; Esposito, A.; Altieri, S.; Battipaglia, G. Fire severity influences ecophysiological responses of Pinus pinaster Ait. Front. Plant Sci. 2019, 10, 539. [Google Scholar] [CrossRef]
- Zalloni, E.; Battipaglia, G.; Cherubini, P.; De Micco, V. Site conditions influence the climate signal of intra-annual density fluctuations in tree rings of Q. ilex L. Ann. Forest Sci. 2018, 75, 68. [Google Scholar] [CrossRef] [Green Version]
- Giuditta, E.; Coenders-Gerrits, A.M.J.; Bogaard, T.A.; Wenninger, J.; Greco, R.; Rutigliano, F.A. Measuring changes in forest floor evaporation after prescribed burning in Southern Italy pine plantations. Agric. Forest Meteorol. 2018, 256–257, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.E. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1989. [Google Scholar]
- Burc, R. (Ed.) USDA Natural Resources Conservation Service Soil Survey Laboratory Methods Manual; Soil Survey Investigations; Report No. 42; Version 4.0.; National Soil Survey Center: Lincoln, NE, USA, 2004.
- Castaldi, S.; Carfora, A.; Fiorentino, A.; Natale, A.; Messere, A.; Miglietta, F.; Cotrufo, M.F. Inhibition of net nitrification activity in a Mediterranean woodland: Possible role of chemicals produced by Arbutus unedo. Plant Soil 2009, 315, 273–283. [Google Scholar] [CrossRef]
- Springer, U.; Klee, J. Prüfung der Leistungsfähigkeit von einigen wichtigeren Verfahren zur Bestimmung des Kohlemstoffs mittels Chromschwefelsäure sowie Vorschlag einer neuen Schnellmethode. Z. Pflanzenernähr. Dang. Bodenk 1954, 64, 1–26. [Google Scholar] [CrossRef]
- Sleutel, S.; De Neve, S.; Singier, B.; Hofman, G. Quantification of organic carbon in soils: A comparison of methodologies and assessment of the carbon content of organic matter. Commun. Soil Sci. Plan. 2007, 38, 2647–2657. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Dequiedt, S.; Saby, N.P.A.; Lelievre, M.; Jolivet, C.; Thioulous, J.; Toutain, B.; Arrouays, D.; Bispo, A.; Lemanceau, P.; Ranjard, L. Biogeographical patterns of soil molecular microbial biomass as influenced by soil characteristics and management. Glob. Ecol. Biogeogr. 2011, 20, 641–652. [Google Scholar] [CrossRef]
- Sundman, V.; Sivelä, S. A comment on the membrane filter technique for estimation of length of fungal hyphae in soil. Soil Biol. Biochem. 1978, 10, 399–401. [Google Scholar] [CrossRef]
- Olson, F.C.W. Quantitative estimates of filamentous algae. Trans. Am. Microsc. Soc. 1950, 69, 272–279. [Google Scholar] [CrossRef]
- Berg, B.; Söderström, B. Fungal biomass and nitrogen in decomposing Scots pine needle litter. Soil Biol. Biochem. 1979, 11, 339–341. [Google Scholar] [CrossRef]
- D’Ascoli, R.; Rutigliano, F.A.; De Pascale, R.A.; Gentile, A.; Virzo De Santo, A. Functional diversity of the microbial community in Mediterranean maquis soils as affected by fires. Int. J. Wildland Fire 2005, 14, 355–363. [Google Scholar] [CrossRef]
- Kieft, T.L.; White, C.S.; Loftin, S.R.; Aguilar, R.; Craig, J.A.; Skaar, D.A. Temporal dynamics in soil carbon and nitrogen resources at a grassland-shrubland ecotone. Ecology 1998, 79, 671–683. [Google Scholar]
- Anderson, N.J.P.E.; Domsch, K.H. A Physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Niro, E.; Marzaioli, R.; De Crescenzo, S.; D’Abrosca, B.; Castaldi, S.; Esposito, A.; Fiorentino, A.; Rutigliano, F.A. Effects of the allelochemical coumarin on plants and soil microbial community. Soil Biol. Biochem. 2016, 95, 30–39. [Google Scholar] [CrossRef]
- Muyzer, G.; Brinkhoff, T.; Nübel, U.; Santegoeds, C.; Schäfer, H.; Wawer, C. Denaturing gradient gel electrophoresis (DGGE) in microbial ecology. In Molecular Microbial Ecology Manual; Akkermans, A.D.L., van Elsas, J.D., Bruijn, F.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 1–27. [Google Scholar]
- Nübel, U.; Engelen, B.; Felske, A.; Snaidr, J.; Wieshuber, A.; Amann, R.I.; Ludwig, W.; Backhaus, H. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol. 1996, 178, 5636–5643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnelli, A.; Ascher, J.; Corti, G.; Ceccherini, M.T.; Nannipieri, P.; Pietramellara, G. Distribution of microbial communities in a forest soil profile investigated by microbial biomass, soil respiration and DGGE of total and extracellular DNA. Soil Biol. Biochem. 2004, 36, 859–868. [Google Scholar] [CrossRef]
- Manefield, M.; Griffiths, R.I.; Leigh, M.B.; Fisher, R.; Whiteley, A.S. Functional and compositional comparison of two activated sludge communities remediating coking effluent. Environ. Microbiol. 2005, 7, 715–722. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rholf, F.J. Biometry: The principles and Practices of Statistic in Biological Research, 3rd ed.; W.H. Freeman & Company: New York, NY, USA, 1994. [Google Scholar]
- Fontúrbel, M.T.; Fernández, C.; Vega, J.A. Prescribed burning versus mechanical treatments as shrubland management options in NW Spain: Mid-term soil microbial response. Appl. Soil Ecol. 2016, 107, 334–346. [Google Scholar] [CrossRef]
- Fioretto, A.; Papa, S.; Pellegrino, A. Effects of fire on soil respiration, ATP content and enzyme activities in Mediterranean maquis. Appl. Veg. Sci. 2005, 8, 13–20. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Castaldi, S.; D’Ascoli, R.; Papa, S.; Carfora, A.; Marzaioli, R.; Fioretto, A. Soil activities related to nitrogen cycle under three plant cover types in Mediterranean environment. Appl. Soil Ecol. 2009, 43, 40–46. [Google Scholar] [CrossRef]
- Meira-Castro, A.C.; Shakesby, R.A.; Marques, J.E.; Doerr, S.H.; Meixedo, J.P.; Teixeira, J.; Chaminé, H.I. Effects of prescribed fire on surface soil in a Pinus pinaster plantation, northern Portugal. Environ. Earth Sci. 2015, 73, 3011–3018. [Google Scholar] [CrossRef] [Green Version]
- Coates, T.A.; Chow, A.T.; Hagan, D.L.; Wang, G.G.; Bridges, W.C., Jr.; Dozier, J.H. Frequent prescribed burning as a long-term practice in longleaf pine forests does not affect detrital chemical composition. J. Environ. Qual. 2017, 46, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Ascher, J.; Ceccherini, M.T.; Pantani, O.L.; Agnelli, A.; Borgoni, F.; Guerri, G.; Nannipieri, P.; Pietramellara, G. Sequential extraction and genetic fingerprinting of a forest soil metagenome. Appl. Soil Ecol. 2009, 42, 176–181. [Google Scholar] [CrossRef]
- Rodríguez, J.; González-Pérez, J.A.; Turmero, A.; Hernández, M.; Ball, A.S.; González-Vila, F.J.; Arias, M.E. Physico-chemical and microbial perturbations of Andalusian pine forest soils following a wildfire. Sci. Total Environ. 2018, 634, 650–660. [Google Scholar] [CrossRef]
- Cookson, W.R.; O’Donnel, A.J.; Grant, C.D.; Grierson, P.F.; Murphy, D.V. Impact of Ecosystem Management on Microbial Community Level Physiological Profiles of Postmining Forest Rehabilitation. Microb. Ecol. 2008, 55, 321–332. [Google Scholar] [CrossRef]
- Capogna, F.; Persiani, A.M.; Maggi, O.; Dowgiallo, G.; Puppi, G.; Manes, F. Effects of different fire intensities on chemical and biological soil components and related feedbacks on a Mediterranean shrub (Phillyrea angustifolia L.). Plant Ecol. 2009, 204, 155–171. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Migliorini, M.; Maggi, O.; D’Ascoli, R.; Fanciulli, P.P.; Persiani, A.M. Dynamics of fungi and fungivorous microarthropods in a Mediterranean maquis soil affected by experimental fire. Eur. J. Soil Biol. 2013, 56, 33–34. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Fierro, A.R.; De Pascale, R.A.; De Marco, A.; De Santo, A.V. Role of fire on soil organic matter turnover and microbial activity in a Mediterranean burned area. Dev. Soil Sci. 2002, 28, 205–215. [Google Scholar]
- Wardle, D.A.; Ghani, A. A critique of the microbial metabolic quotient (qCO2) as a bioindicator of disturbance and ecosystem development. Soil Biol. Biochem. 1995, 27, 1601–1610. [Google Scholar] [CrossRef]




| P. pinea | P. pinaster | |
|---|---|---|
| Stand Structure | ||
| Tree density (n. ha−1) | 954 (±178) | 1294 (±243) |
| Tree cover (%) | 65 (±7.1) | 62.3 (±5.9) |
| Tree high (m) | 13.5 (±2.1) | 11.7 (±0.7) |
| Shrub cover (%) | 70.0 (±14.1) | 37.1 (±24.6) |
| Shrub high (m) | 1.8 (±3.5) | 1.9 (±1.2) |
| Herb cover (%) | 62.5 (±12.6) | 2.1 (±1.7) |
| Herb high (cm) | 32.5 (±5.0) | 15.0 (±0.7) |
| Fuel load (t ha−1) | ||
| Litter (<6 mm) | 6.4 (±1.1) | 14.8 (±1.7) |
| Woody litter (6–25 mm) | 1.4 (±1.0) | 2.7 (±2.3) |
| Herb fuels | 0.54 (±0.21) | Not analysed |
| Weather data 2 | ||
| Air temperature (°C) | 18 (±0.65) | 16 (±0.57) |
| Relative humidity (%) | 54 (±4.1) | 52 (±3.7) |
| Litter moisture (%) | 32 (±3.8) | 38 (±4.1) |
| Wind speed (km h−1) | 2.7 (±0.9) | 4.2 (±1.8) |
| Number of days since rain before the treatment | 8 | 17 |
| Fire behavior | ||
| Ignition pattern | Backfire | Backfire |
| Flame length (m) | <0.5 | <1 |
| Rate of spread (m min−1) | 0.14 (±0.04) | 0.19 (±0.03) |
| Fireline intensity (kW m−1) | <50 | <150 |
| Litter mean maximum temperature (°C) | 347 (±202) | 574 (±205) |
| F-layer mean maximum temperature (°C) | 95 (±136) | 438 (±97) |
| Mean residence of temperature (s) above 100 °C in the litter | 139 (±90) | 230 (±42) |
| Mean residence of temperature (s) above 100 °C under F-layer | 30 (±67) | 180 (±70) |
| (A) Time after Burning (Sampling Date) | pH | Ntot (g kg−1 d.w.) | NH4+-N (mg kg−1 d.w.) | NO3−-N (mg kg−1 d.w.) |
| 3 h (12 March 2014) | ||||
| Control | 7.2 (±0.6) | 2.0 (±0.5) | 1.6 (±1.1) | 4.6 (±1.3) |
| Burned | 6.6 (±0.8) | 2.6 (±0.7) | 4.1 (±2.8) | 7.0 (±2.1) |
| 33 d (14 April 2014) | ||||
| Control | 6.8 (±0.6) | 2.2 (±0.8) | 11.8 (±2.0) | 6.2 (±2.5) |
| Burned | 6.4 (±0.5) | 3.1 (±0.9) | 13.7 (±2.6) | 5.8 (±1.8) |
| 89 d (9 June 2014) | ||||
| Control | 6.5 (±0.7) | 2.7 (±0.9) | 7.6 (±0.7) | 7.6 (±3.6) a |
| Burned | 6.8 (±0.5) | 2.9 (±0.7) | 9.4 (±3.9) | 48.3 (±23.4) b |
| 189 d (17 September 2014) | ||||
| Control | 6.7 (±0.4) | 2.1 (±0.3) | 0.9 (± 0.3) | 24.0 (±7.7) a |
| Burned | 6.5 (±0.5) | 2.6 (±0.7) | 1.2 (±0.4) | 54.3 (±26.2) b |
| 363 d (10 March 2015) | ||||
| Control | 6.7 (±0.4) | 2.2 (±0.6) | 13.7 (±2.2) | 2.4 (±0.5) |
| Burned | 6.5 (±0.5) | 2.4 (±0.6) | 15.9 (±2.3) | 2.4 (±0.9) |
| Burning | N.S. | p < 0.05 | p = 0.001 | p < 0.001 |
| Sampling time | N.S. | N.S. | p < 0.001 | p < 0.001 |
| (B) Time after Burning (Sampling Date) | pH | Ntot (g kg−1 d.w.) | NH4+-N (mg kg−1 d.w.) | NO3−-N (mg kg−1 d.w.) |
| 3 h (21 March 2014) | ||||
| Control | 5.6 (±0.5) | 4.5 (±3.0) | 1.3 (±1.0) | 1.8 (±0.3) |
| Burned | 6.2 (±0.4) | 4.9 (±4.8) | 2.2 (±1.5) | 1.7 (±0.1) |
| 32 d (22 April 2014) | ||||
| Control | 6.2 (±0.5) | 4.9 (±3.2) | 31.5 (±15.6) a | 4.0 (±1.1) |
| Burned | 6.4 (±0.2) | 1.2 (±0.4) | 14.3 (±5.0) b | 4.1 (±0.4) |
| 87 d (16 June 2014) | ||||
| Control | 6.1 (±0.5) | 1.5 (±0.9) | 2.0 (±0.2) | 1.2 (±0.2) |
| Burned | 6.3 (±0.5) | 2.5 (±1.8) | 2.1 (±0.2) | 1.1 (±0.1) |
| 182 d (19 September 2014) | ||||
| Control | 6.0 (±0.5) | 7.4 (±3.3) | 3.4 (±1.6) a | 2.9 (±0.7) |
| Burned | 6.2 (±0.3) | 7.5 (±4.0) | 1.7 (±0.5) b | 2.4 (±0.5) |
| 371 d (27 March 2015) | ||||
| Control | 6.0 (±0.5) | 7.4 (±3.6) | 32.3 (±8.7) | 3.4 (±1.0) |
| Burned | 6.2 (±0.3) | 7.4 (±2.9) | 28.4 (±12.3) | 2.6 (±1.0) |
| Burning | p < 0.05 | N.S. | N.S. | N.S. |
| Sampling time | N.S. | p < 0.001 | p < 0.001 | p < 0.001 |
| (A) Time after Burning (Sampling Date) | F-Layer | S-Layer | ||||
| WC (%) | Corg (g kg−1 d.w.) | Cext (g kg−1 d.w.) | WC (%) | Corg (g kg−1 d.w.) | Cext (g kg−1 d.w.) | |
| 3 h (12 March 2014) | ||||||
| Control | 164 (±44) | 505.8 (±46.9) | 2.6 (±0.7) | 25 (±7) | 46.5 (±4.6) | 0.2 (±0.1) |
| Burned | 182 (±20) | 503.3(±42.8) | 2.6 (±0.4) | 31 (±8) | 48.8 (±11.1) | 0.3 (±0.04) |
| 33 d (14 April 2014) | ||||||
| Control | 180 (±27) | 482.5 (±27.6) | 2.7 (±0.3) | 22 (±7) | 48.9 (±16.4) | 0.2 (±0.03) |
| Burned | 168 (±9) | 481.8 (±51.5) | 2.5 (±0.4) | 25 (±14) | 51.9 (±12.3) | 0.2 (±0.1) |
| 89 d (9 June 2014) | ||||||
| Control | 34 (±10) | 422.2 (±60.3) | 1.1 (±0.3) | 15 (±11) | 45.1 (±5.4) | 0.1 (±0.04) |
| Burned | 35 (±11) | 367.7 (±37.1) | 1.9 (±0.3) | 18 (±14) | 46.1 (±7.7) | 0.2 (±0.04) |
| 189 d (17 September 2014) | ||||||
| Control | 182 (±81) | 441.9 (±30.7) | 2.4 (±0.9) | 14 (±3) | 52.4 (±7.4) | 0.2 (±0.1) |
| Burned | 159 (±38) | 451.9 (±50.3) | 2.2 (±0.5) | 15 (±3) | 53.2 (±12.7) | 0.2 (±0.04) |
| 363 d (10 March 2015) | ||||||
| Control | 172 (±21) | 359.7 (±50.2) | 2.8 (±0.7) a | 29 (±13) | 48.5 (±9.5) | 0.3 (±0.02) |
| Burned | 154 (±41) | 337.6 (±48.6) | 4.4 (±1.0) b | 28 (±11) | 49.6 (±9.5) | 0.3 (±0.1) |
| Burning | N.S. | N.S. | p < 0.05 | N.S. | N.S. | N.S. |
| Sampling time | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | N.S. | p < 0.001 |
| (B) Time after Burning (Sampling Date) | F-layer | S-layer | ||||
| WC (%) | Corg (g kg−1 d.w.) | Cext (g kg−1 d.w.) | WC (%) | Corg (g kg−1 d.w.) | Cext (g kg−1 d.w.) | |
| 3 h (21 March 2014) | ||||||
| Control | 267 (±92) a | 413.9 (±39.1) | 2.6 (±0.7) | 54 (±14) | 82.8 (±5.9) | 0.3 (±0.1) |
| Burned | 147 (±41) b | 404.0 (±36.5) | 2.7 (±1.0) | 31 (±13) | 78.0 (±4.4) | 0.2 (±0.1) |
| 32 d (22 April 2014) | ||||||
| Control | 258 (±76) a | 426.6 (±45.4) | 4.3 (±1.4) | 58 (±15)a | 79.0 (±8.2) | 0.3 (±0.1) |
| Burned | 179 (±33) b | 421.1 (±36.1) | 2.9 (±0.8) | 21 (±6)b | 75.7 (±15.3) | 0.2 (±0.1) |
| 87 d (16 June 2014) | ||||||
| Control | 135 (±40) | 407.4 (±35.7) | 2.5 (±0.8) | 19 (±6) | 78.5 (±11.7) | 0.1 (±0.1) |
| Burned | 97 (±34) | 400.0 (±53.3) | 1.9 (±0.3) | 23 (±9) | 84.9 (±14.9) | 0.2 (±0.03) |
| 182 d (19 September 2014) | ||||||
| Control | 292 (±32) | 430.3 (±46.3) | 4.7 (±1.0) | 162 (±32) | 75.8 (±12.7) | 0.5 (±0.2) |
| Burned | 264 (±41) | 429.3 (±47.1) | 4.4 (±1.1) | 140 (±47) | 77.2 (±4.6) | 0.5 (±0.2) |
| 371 d (27 March 2015) | ||||||
| Control | 247 (±55) | 426.9 (±36.3) | 5.5 (±1.3) | 172 (±80) | 79.6 (±12.8) | 0.5 (±0.1) |
| Burned | 256 (±49) | 426.6 (±48.7) | 4.3 (±0.9) | 109 (±86) | 78.3 (±9.1) | 0.3 (±0.1) |
| Burning | p < 0.001 | N.S. | p < 0.05 | p < 0.001 | N.S. | p < 0.05 |
| Sampling time | p < 0.001 | N.S. | p < 0.001 | p < 0.001 | N.S. | p < 0.001 |
| (A) Time after Burning (Sampling Date) | F-Layer | S-Layer | ||||
| Richness (Band Number) | Shannon Index | Eveness Index | Richness (Band Number) | Shannon Index | Eveness Index | |
| 3 h (12 March 2014) | ||||||
| Control | 27.0 (±0.0) | 3.1 (±0.1) | 0.96 (±0.00) | 39 (±0.0) | 3.3 (±0.1) | 0.91 (±0.02) |
| Burned | 27.5 (±2.1) | 3.1 (±0.0) | 0.92 (±0.02) | 36 (±4.2) | 3.3 (±0.1) | 0.93 (±0.00) |
| 33 d (14 April 2014) | ||||||
| Control | 29.0 (±2.8) | 3.2 (±0.1) | 0.94 (±0.03) | 39.0 (±4.2) | 3.4 (±0.2) | 0.93 (±0.02) |
| Burned | 26.5 (±4.9) | 3.0 (±0.1) | 0.94 (±0.00) | 35.0 (±1.4) | 3.4 (±0.0) | 0.94 (±0.02) |
| 189 d (17 September 2014) | ||||||
| Control | 24.0 (±4.2) | 3.0 (±0.1) | 0.96 (±0.00) | 39.0 (±1.4) | 3.5 (±0.1) | 0.95 (±0.05) |
| Burned | 25.5(±9.2) | 3.0 (±0.4) | 0.93 (±0.02) | 33.0(±0.0) | 3.3 (±0.1) | 0.94 (±0.02) |
| 363 d (10 March 2015) | ||||||
| Control | 21.5 (±0.7) | 2.9 (±0.0) | 0.94 (±0.01) | 39.5 (±3.5) | 3.4 (±0.0) | 0.93 (±0.01) |
| Burned | 22.0 (±2.8) | 2.7 (±0.1) | 0.90 (±0.07) | 38.0 (±0.0) | 3.4 (±0.0) | 0.94 (±0.01) |
| Burning | N.S. | N.S. | N.S. | P < 0.05 | N.S. | N.S. |
| Sampling time | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| (B) Time after Burning (Sampling Date) | F-layer | S-layer | ||||
| Richness (band number) | Shannon index | Eveness index | Richness (band number) | Shannon index | Eveness Index | |
| 3 h (21 March 2014) | ||||||
| Control | 39.5 (±2.1) | 3.6 (±0.1) | 0.97 (±0.02) | 40.0 (±5.7) | 3.5 (±0.1) | 0.96 (±0.00) |
| Burned | 36.5 (±0.7) | 3.5 (±0.1) | 0.96 (±0.01) | 37.0 (±1.4) | 3.5 (±0.1) | 0.96 (±0.02) |
| 32 d (22 April 2014) | ||||||
| Control | 38.0 (±4.2) | 3.5 (±0.1) | 0.97 (±0.01) | 36.0 (±2.8) | 3.4 (±0.2) | 0.95 (±0.03) |
| Burned | 36.0 (±2.8) | 3.5 (±0.1) | 0.97 (±0.01) | 38.0 (±1.4) | 3.5 (±0.0) | 0.95 (±0.01) |
| 182 d (19 September 2014) | ||||||
| Control | 44.0 (±2.82) | 3.7 (±0.1) | 0.98 (±0.01) | 38.0 (±7.1) | 3.5 (±0.1) | 0.96 (±0.01) |
| Burned | 41.5 (±3.5) | 3.6 (±0.1) | 0.96 (±0.01) | 29.0 (±7.1) | 3.2 (±0.2) | 0.96 (±0.01) |
| 371 d (27 March 2015) | ||||||
| Control | 43.5 (±0.7) | 3.7 (±0.0) | 0.97 (±0.00) | 43.5 (±2.1) | 3.7 (±0.0) | 0.98 (±0.00) |
| Burned | 42.0 (±0.0) | 3.5 (±0.1) | 0.94 (±0.01) | 42.0 (±5.7) | 3.6 (±0.1) | 0.96 (±0.00) |
| Burning | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Sampling time | p < 0.05 | N.S. | N.S. | N.S. | N.S. | N.S. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuditta, E.; Marzaioli, R.; Esposito, A.; Ascoli, D.; Stinca, A.; Mazzoleni, S.; Rutigliano, F.A. Soil Microbial Diversity, Biomass, and Activity in Two Pine Plantations of Southern Italy Treated with Prescribed Burning. Forests 2020, 11, 19. https://doi.org/10.3390/f11010019
Giuditta E, Marzaioli R, Esposito A, Ascoli D, Stinca A, Mazzoleni S, Rutigliano FA. Soil Microbial Diversity, Biomass, and Activity in Two Pine Plantations of Southern Italy Treated with Prescribed Burning. Forests. 2020; 11(1):19. https://doi.org/10.3390/f11010019
Chicago/Turabian StyleGiuditta, Elisabetta, Rossana Marzaioli, Assunta Esposito, Davide Ascoli, Adriano Stinca, Stefano Mazzoleni, and Flora A. Rutigliano. 2020. "Soil Microbial Diversity, Biomass, and Activity in Two Pine Plantations of Southern Italy Treated with Prescribed Burning" Forests 11, no. 1: 19. https://doi.org/10.3390/f11010019
APA StyleGiuditta, E., Marzaioli, R., Esposito, A., Ascoli, D., Stinca, A., Mazzoleni, S., & Rutigliano, F. A. (2020). Soil Microbial Diversity, Biomass, and Activity in Two Pine Plantations of Southern Italy Treated with Prescribed Burning. Forests, 11(1), 19. https://doi.org/10.3390/f11010019