Effects of Elevated Temperature and Ozone in Brassica juncea L.: Growth, Physiology, and ROS Accumulation
Abstract
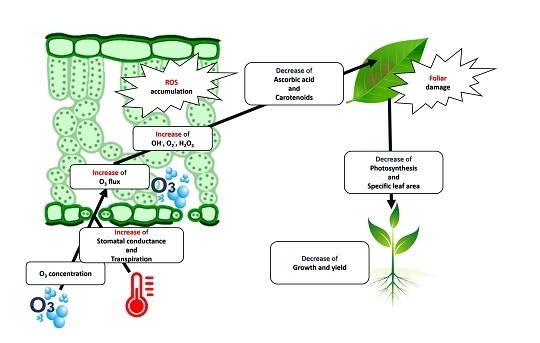
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Experimental Treatments
2.3. Plant Injury Indices
2.4. Measurement of Gas Exchange Parameters and Calculation of Ozone Flux
2.5. Measurement of Carotenoids and Ascorbic Acid
2.6. Measurement of Lipid Peroxidation and Reactive Oxygen Species
2.7. Measurement of Growth Characteristics
2.8. Statistical Analyses
3. Results
3.1. Plant Injury Indices
3.2. Growth Characteristics
3.3. Gas Exchange Characteristics and O3 Flux
3.4. Carotenoid and Ascorbic Acid Contents
3.5. Lipid Peroxidation and Reactive Oxygen Species (ROS) Accumulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Ainsworth, E.A.; Rogers, A.; Leakey, A.D. Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol. 2008, 147, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, I.F.; Wrigley, C.W. Heat tolerance in temperate cereals: An overview. Funct. Plant Biol. 1994, 21, 695–703. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Effects of temperature and watering regime on growth, gas exchange and abscisic acid content of canola (Brassica napus) seedlings. Environ. Exp. Bot. 2012, 75, 107–113. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Bokszczanin, K.L.; Fragkostefanakis, S.; Bostan, H.; Bovy, A.; Chaturvedi, P.; Chiusano, M.L.; Firon, N.; Iannacone, R.; Jegadeesan, S.; Klaczynskid, K. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front. Plant Sci. 2013, 4, 315. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Gong, Q.; Li, P.; Ma, S. Unraveling abiotic stress tolerance mechanisms–getting genomics going. Curr. Opin. Plant Biol. 2006, 9, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Yao, X. Air pollution in mega cities in China. Atmos. Environ. 2008, 42, 1–42. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, D.K.; Gan, J.; Park, C.; Kim, S.; Sung, S.; Jung, T.Y.; Hong, S.C. Effects of climate change and ozone concentration on the net primary productivity of forests in South Korea. Forests 2018, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, T.; Ohara, T.; Sudo, K.; Akimoto, H. The relative importance of various source regions on East Asian surface ozone. Atmos. Chem. Phys. 2010, 10, 11305–11322. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Jacob, D.J.; Liao, H.; Shen, L.; Zhang, Q.; Bates, K.H. Anthropogenic drivers of 2013–2017 trends in summer surface ozone in China. Proc. Natl. Acad. Sci. USA 2019, 116, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Dizengremel, P.; Le Thiec, D.; Hasenfratz-Sauder, M.; Vaultier, M.; Bagard, M.; Jolivet, Y. Metabolic-dependent changes in plant cell redox power after ozone exposure. Plant Biol. 2009, 11, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Renaut, J.; Bohler, S.; Hausman, J.; Hoffmann, L.; Sergeant, K.; Ahsan, N.; Jolivet, Y.; Dizengremel, P. The impact of atmospheric composition on plants: A case study of ozone and poplar. Mass Spectrom. Rev. 2009, 28, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Krupa, S.; McGrath, M.T.; Andersen, C.P.; Booker, F.L.; Burkey, K.O.; Chappelka, A.H.; Chevone, B.I.; Pell, E.J.; Zilinskas, B.A. Ambient ozone and plant health. Plant Dis. 2001, 85, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Picchi, V.; Monga, R.; Marzuoli, R.; Gerosa, G.; Faoro, F. The ozone-like syndrome in durum wheat (Triticum durum Desf.): Mechanisms underlying the different symptomatic responses of two sensitive cultivars. Plant Physiol. Biochem. 2017, 112, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Laisk, A.; Kull, O.; Moldau, H. Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol. 1989, 90, 1163–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaspers, P.; Kangasjärvi, J. Reactive oxygen species in abiotic stress signaling. Physiol. Plant 2010, 138, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Yendrek, C.R.; Sitch, S.; Collins, W.J.; Emberson, L.D. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu. Rev. Plant Biol. 2012, 63, 637–661. [Google Scholar] [CrossRef] [Green Version]
- Moura, B.B.; Alves, E.S.; Marabesi, M.A.; De Souza, S.R.; Schaub, M.; Vollenweider, P. Ozone affects leaf physiology and causes injury to foliage of native tree species from the tropical Atlantic Forest of southern Brazil. Sci. Total Environ. 2018, 610, 912–925. [Google Scholar] [CrossRef]
- Frenck, G.; Van der Linden, L.; Mikkelsen, T.N.; Brix, H.; Jørgensen, R.B. Increased [CO2] does not compensate for negative effects on yield caused by higher temperature and [O3] in Brassica napus L. Eur. J. Agron. 2011, 35, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Hansen, E.M.; Hauggaard-Nielsen, H.; Launay, M.; Rose, P.; Mikkelsen, T.N. The impact of ozone exposure, temperature and CO2 on the growth and yield of three spring wheat varieties. Environ. Exp. Bot. 2019, 168, 103868. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. Annual Report of Air Quality in Korea 2016; National Institute of Environmental Research: Incheon, Korea, 2017; pp. 17–26. [Google Scholar]
- Feng, Z.; Sun, J.; Wan, W.; Hu, E.; Calatayud, V. Evidence of widespread ozone-induced visible injury on plants in Beijing, China. Environ. Pollut. 2014, 193, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, V.; García-Breijo, F.J.; Cervero, J.; Reig-Armiñana, J.; Sanz, M.J. Physiological, anatomical and biomass partitioning responses to ozone in the Mediterranean endemic plant Lamottea dianae. Ecotoxicol. Environ. Saf. 2011, 74, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Fiscus, E.L.; Reid, C.D.; Miller, J.E.; Heagle, A.S. Elevated CO2 reduces O3 flux and O3-induced yield losses in soybeans: Possible implications for elevated CO2 studies. J. Exp. Bot. 1997, 48, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Moldau, H.; Bichele, I. Plasmalemma protection by the apoplast as assessed from above-zero ozone concentrations in leaf intercellular air spaces. Planta 2002, 214, 484–487. [Google Scholar] [CrossRef]
- Li, S.; Harley, P.C.; Niinemets, Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant Cell Environ. 2017, 40, 1984–2003. [Google Scholar] [CrossRef] [Green Version]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Keller, T.; Schwager, H. Air pollution and ascorbic acid. For. Pathol. 1977, 7, 338–350. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Doke, N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol. Plant Pathol. 1983, 23, 345–357. [Google Scholar] [CrossRef]
- Tiedemann, A.V. Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol. Mol. Plant Pathol. 1997, 50, 151–166. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Curves. The Functional Approach to Plant Growth Analysis; Edward Arnold Ltd.: London, UK, 1990; pp. 98–120. [Google Scholar]
- Zhang, L.; Xiao, S.; Chen, Y.J.; Xu, H.; Li, Y.G.; Zhang, Y.W.; Luan, F.S. Ozone sensitivity of four Pakchoi cultivars with different leaf colors: Physiological and biochemical mechanisms. Photosynthetica 2017, 55, 478–490. [Google Scholar] [CrossRef]
- Reich, P.B. Quantifying plant response to ozone: A unifying theory. Tree Physiol. 1987, 3, 63–91. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, I.; Elvira, S.; Calatayud, V.; Calvo, E.; Aparicio, P.; Sánchez, M.; Alonso, R.; Bermejo, V.B. Ozone effects on the physiology and marketable biomass of leafy vegetables under Mediterranean conditions: Spinach (Spinacia Oleracea L.) and Swiss chard (Beta Vulgaris L. Var. cycla). Agric. Ecosyst. Environ. 2016, 235, 215–228. [Google Scholar] [CrossRef]
- Law, R.D.; Crafts-Brandner, S.J. Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1, 5-bisphosphate Carboxylase/Oxygenase. Plant Physiol. 1999, 120, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tallman, G. Are diurnal patterns of stomatal movement the result of alternating metabolism of endogenous guard cell ABA and accumulation of ABA delivered to the apoplast around guard cells by transpiration? J. Exp. Bot. 2004, 55, 1963–1976. [Google Scholar] [CrossRef] [Green Version]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Apgaua, D.M.; Tng, D.Y.; Forbes, S.J.; Ishida, Y.F.; Vogado, N.O.; Cernusak, L.A.; Laurance, S.G. Elevated temperature and CO2 cause differential growth stimulation and drought survival responses in eucalypt species from contrasting habitats. Tree Physiol. 2019, 39, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Agrawal, M.; Marshall, F.M. Seasonal variations in adaptational strategies of Beta vulgaris L. plants in response to ambient air pollution: Biomass allocation, yield and nutritional quality. Trop. Ecol. 2010, 51, 353–363. [Google Scholar]
- Kumari, S.; Agrawal, M. Growth, yield and quality attributes of a tropical potato variety (Solanum tuberosum L. cv. Kufri chandramukhi) under ambient and elevated carbon dioxide and ozone and their interactions. Ecotoxicol. Environ. Saf. 2014, 101, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Rosbakh, S.; Römermann, C.; Poschlod, P. Specific leaf area correlates with temperature: New evidence of trait variation at the population, species and community levels. Alpine Bot. 2015, 125, 79–86. [Google Scholar] [CrossRef]
- Biswas, D.K.; Xu, H.; Li, Y.G.; Ma, B.L.; Jiang, G.M. Modification of photosynthesis and growth responses to elevated CO2 by ozone in two cultivars of winter wheat with different years of release. J. Exp. Bot. 2013, 64, 1485–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savaldi-Goldstein, S.; Peto, C.; Chory, J. The epidermis both drives and restricts plant shoot growth. Nature 2007, 446, 199. [Google Scholar] [CrossRef]
- Riikonen, J.; Percy, K.E.; Kivimäenpää, M.; Kubiske, M.E.; Nelson, N.D.; Vapaavuori, E.; Karnosky, D.F. Leaf size and surface characteristics of Betula papyrifera exposed to elevated CO2 and O3. Environ. Pollut. 2010, 158, 1029–1035. [Google Scholar] [CrossRef]
- Ashmore, M.R. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005, 28, 949–964. [Google Scholar] [CrossRef]
- Fiscus, E.L.; Booker, F.L.; Burkey, K.O. Crop responses to ozone: Uptake, modes of action, carbon assimilation and partitioning. Plant Cell Environ. 2005, 28, 997–1011. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Z.; Wang, X.; Niu, J. Elevated ozone negatively affects photosynthesis of current-year leaves but not previous-year leaves in evergreen Cyclobalanopsis glauca seedlings. Environ. Pollut. 2014, 184, 676–681. [Google Scholar] [CrossRef]
- Gago, J.; Carriquí, M.; Nadal, M.; Clemente-Moreno, M.J.; Coopman, R.E.; Fernie, A.R.; Flexas, J. Photosynthesis optimized across land plant phylogeny. Trends Plant Sci. 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Pell, E.; Schlagnhaufer, C.D.; Arteca, R.N. Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiol. Plant 1997, 100, 264–273. [Google Scholar] [CrossRef]
- Morgan, P.B.; Ainsworth, E.A.; Long, S.P. How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant Cell Environ. 2003, 26, 1317–1328. [Google Scholar] [CrossRef]
- Morgan, P.B.; Bernacchi, C.J.; Ort, D.R.; Long, S.P. An in vivo analysis of the effect of season-long open-air elevation of ozone to anticipated 2050 levels on photosynthesis in soybean. Plant Physiol. 2004, 135, 2348–2357. [Google Scholar] [CrossRef] [Green Version]
- Posch, B.C.; Kariyawasam, B.C.; Bramley, H.; Coast, O.; Richards, R.A.; Reynolds, M.P.; Trethowan, R.; Atkin, O.K. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J. Exp. Bot. 2019, 70, 5051–5069. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G.; Matyssek, R. Linking ozone uptake and defense towards a mechanistic risk assessment for forest trees. New Phytol. 2007, 174, 7–9. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Leakey, A.D.; Heady, L.E.; Morgan, P.B.; Dohleman, F.G.; McGrath, J.M.; Gillespie, K.M.; Wittig, V.E.; Rogers, A.; Long, S.P. Hourly and seasonal variation in photosynthesis and stomatal conductance of soybean grown at future CO2 and ozone concentrations for 3 years under fully open-air field conditions. Plant Cell Environ. 2006, 29, 2077–2090. [Google Scholar] [CrossRef]
- Uddling, J.; Hogg, A.J.; Teclaw, R.M.; Carroll, M.A.; Ellsworth, D.S. Stomatal uptake of O3 in aspen and aspen-birch forests under free-air CO2 and O3 enrichment. Environ. Pollut. 2010, 158, 2023–2031. [Google Scholar] [CrossRef]
- Hartikainen, K.; Riikonen, J.; Nerg, A.; Kivimäenpää, M.; Ahonen, V.; Tervahauta, A.; Kärenlampi, S.; Mäenpää, M.; Rousi, M.; Kontunen-Soppela, S. Impact of elevated temperature and ozone on the emission of volatile organic compounds and gas exchange of silver birch (Betula pendula Roth). Environ. Exp. Bot. 2012, 84, 33–43. [Google Scholar] [CrossRef]
- Rashid, M.A.; Andersen, M.N.; Wollenweber, B.; Zhang, X.; Olesen, J.E. Acclimation to higher VPD and temperature minimized negative effects on assimilation and grain yield of wheat. Agric. For. Meteorol. 2018, 248, 119–129. [Google Scholar] [CrossRef]
- Kumari, S.; Agrawal, M.; Tiwari, S. Impact of elevated CO2 and elevated O3 on Beta vulgaris L.: Pigments, metabolites, antioxidants, growth and yield. Environ. Pollut. 2013, 174, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Agrawal, M.; Singh, A. Effects of ambient and elevated CO2 and ozone on physiological characteristics, antioxidative defense system and metabolites of potato in relation to ozone flux. Environ. Exp. Bot. 2015, 109, 276–287. [Google Scholar] [CrossRef]
- Calatayud, A.; Barreno, E. Response to ozone in two lettuce varieties on chlorophyll a fluorescence, photosynthetic pigments and lipid peroxidation. Plant Physiol. Biochem. 2004, 42, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Ann. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Properties and functions of glutathione reductase in plants. Physiol. Plant 1989, 77, 449–456. [Google Scholar] [CrossRef]
- Fehsenfeld, F.; Calvert, J.; Fall, R.; Goldan, P.; Guenther, A.B.; Hewitt, C.N.; Lamb, B.; Liu, S.; Trainer, M.; Westberg, H. Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Glob. Biogeochem. Cycles 1992, 6, 389–430. [Google Scholar] [CrossRef]
- Ghirardo, A.; Koch, K.; Taipale, R.; Zimmer, I.; Schnitzler, J.; Rinne, J. Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13CO2 labelling and PTR-MS analysis. Plant Cell Environ. 2010, 33, 781–792. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Fujiwara, Y.; Kondo, T. Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic. Biol. Med. 2006, 40, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M.; Toyomizu, M. Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS ONE 2013, 8, e64412. [Google Scholar] [CrossRef]
- Grimes, H.D.; Perkins, K.K.; Boss, W.F. Ozone degrades into hydroxyl radical under physiological conditions: A spin trapping study. Plant Physiol. 1983, 72, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Ahlfors, R.; Brosché, M.; Kollist, H.; Kangasjärvi, J. Nitric oxide modulates ozone-induced cell death, hormone biosynthesis and gene expression in Arabidopsis thaliana. Plant J. 2009, 58, 1–12. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide-and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Kanematsu, S.; Asada, K. Intra-and extra-cellular localization of “cytosolic” CuZn-superoxide dismutase in spinach leaf and hypocotyl. Plant Cell Physiol. 1996, 37, 790–799. [Google Scholar] [CrossRef] [Green Version]






| Parameters | Control | Elevated Temp. | Elevated O3 | Elevated Temp. × O3 |
|---|---|---|---|---|
| Total fresh weight (g) | 77.85 ± 1.79 a | 54.85 ± 1.85 b | 44.51 ± 1.77 c | 32.32 ± 1.43 d |
| Total dry weight (g) | 8.82 ± 0.49 a | 6.99 ± 0.07 b | 4.49 ± 0.19 c | 3.37 ± 0.27 c |
| Shoot dry weight (g) | 4.73 ± 0.19 a | 4.55 ± 0.18 a | 2.91 ± 0.13 b | 2.62 ± 0.14 b |
| Root dry weight (g) | 4.08 ± 0.51 a | 2.45 ± 0.19 b | 1.58 ± 0.11 bc | 0.75 ± 0.16 c |
| Shoot:root ratio (SRR) | 1.02 ± 0.13 b | 1.95 ± 0.12 b | 2.11 ± 0.16 b | 4.89 ± 1.12 a |
| Specific leaf area (SLA) (cm2 g−1) | 273.43 ± 7.30 c | 312.72 ± 10.58 bc | 355.38 ± 12.99 ab | 369.77 ± 14.93 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.K.; Woo, S.Y.; Kwak, M.J.; Park, S.H.; Kim, H.D.; Lim, Y.J.; Park, J.H.; Lee, K.A. Effects of Elevated Temperature and Ozone in Brassica juncea L.: Growth, Physiology, and ROS Accumulation. Forests 2020, 11, 68. https://doi.org/10.3390/f11010068
Lee JK, Woo SY, Kwak MJ, Park SH, Kim HD, Lim YJ, Park JH, Lee KA. Effects of Elevated Temperature and Ozone in Brassica juncea L.: Growth, Physiology, and ROS Accumulation. Forests. 2020; 11(1):68. https://doi.org/10.3390/f11010068
Chicago/Turabian StyleLee, Jong Kyu, Su Young Woo, Myeong Ja Kwak, Sang Hee Park, Han Dong Kim, Yea Ji Lim, Jeong Ho Park, and Keum Ah Lee. 2020. "Effects of Elevated Temperature and Ozone in Brassica juncea L.: Growth, Physiology, and ROS Accumulation" Forests 11, no. 1: 68. https://doi.org/10.3390/f11010068
APA StyleLee, J. K., Woo, S. Y., Kwak, M. J., Park, S. H., Kim, H. D., Lim, Y. J., Park, J. H., & Lee, K. A. (2020). Effects of Elevated Temperature and Ozone in Brassica juncea L.: Growth, Physiology, and ROS Accumulation. Forests, 11(1), 68. https://doi.org/10.3390/f11010068