Synergetic Roles of Mangrove Vegetation on Sediment Accretion in Coastal Mangrove Plantations in Central Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Points
2.3. Vegetation Structure
2.4. Quantitative Characteristics of Pneumatophores and Belowground Roots
2.5. Sedimentation Rates
2.6. Elevation Changes
2.7. Environmental Factors
2.8. Data Analysis
3. Results
3.1. Vegetation Structure: Mangrove Trees and Seedlings
3.2. Quantitative Characteristics of Pneumatophores and Belowground Roots of A. alba
3.3. Sedimentation Rates and Elevation Changes
3.4. Environmental Factors
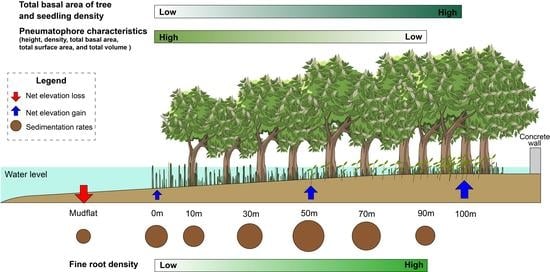
3.5. Relationships among Mangrove Vegetation Structure, Sedimentation Regimes, and Elevation Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koh, H.; Teh, S.; Kh’Ng, X.; Barizan, R.R. Mangrove forests: Protection against and resilience to coastal disturbances. J. Trop. For. Sci. 2018, 30, 446–460. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Gu, J.-D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegrad. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; MacKenzie, R.A. Impacts of climate change on mangrove ecosystems: A region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef] [Green Version]
- Willemsen, P.; Horstman, E.; Borsje, B.; Friess, D.; Dohmen-Janssen, C. Sensitivity of the sediment trapping capacity of an estuarine mangrove forest. Geomorphology 2016, 273, 189–201. [Google Scholar] [CrossRef]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Alongi, D.M. Global significance of mangrove blue carbon in climate change mitigation. Sci 2020, 2, 57. [Google Scholar] [CrossRef]
- Taillardat, P.; Friess, D.A.; Lupascu, M. Mangrove blue carbon strategies for climate change mitigation are most effective at the national scale. Biol. Lett. 2018, 14, 20180251. [Google Scholar] [CrossRef] [Green Version]
- Krauss, K.W.; McKee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R.; Chen, L. How mangrove forests adjust to rising sea level. New Phytol. 2014, 202, 19–34. [Google Scholar] [CrossRef] [Green Version]
- Ellison, A. Mangrove restoration: Do we know enough? Restor. Ecol. 2000, 8, 219–229. [Google Scholar] [CrossRef]
- Field, C. Rehabilitation of mangrove ecosystems: An overview. Mar. Pollut. Bull. 1999, 37, 383–392. [Google Scholar] [CrossRef]
- Ellison, A.M.; Felson, A.J.; Friess, D.A. Mangrove rehabilitation and restoration as experimental adaptive management. Front. Mar. Sci. 2020, 7, 327–345. [Google Scholar] [CrossRef]
- Das, S. Does mangrove plantation reduce coastal erosion? Assessment from the west coast of India. Reg. Environ. Chang. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, X.; Tam, N.F.-Y.; Lu, W.; Luo, Z.; Du, X.; Wang, J. Comparing carbon sequestration and stand structure of monoculture and mixed mangrove plantations of Sonneratia caseolaris and S. apetala in Southern China. For. Ecol. Manag. 2012, 284, 222–229. [Google Scholar] [CrossRef]
- Chow, J. Mangrove management for climate change adaptation and sustainable development in coastal zones. J. Sustain. For. 2017, 37, 139–156. [Google Scholar] [CrossRef]
- Chen, L.; Lin, Q.; Krauss, K.W.; Zhang, Y.; Cormier, N.; Yang, Q. Forest thinning in the seaward fringe speeds up surface elevation increment and carbon accumulation in managed mangrove forests. J. Appl. Ecol. 2021, 58, 1899–1909. [Google Scholar] [CrossRef]
- Krauss, K.; Allen, J.; Cahoon, D. Differential rates of vertical accretion and elevation change among aerial root types in Micronesian mangrove forests. Estuar. Coast. Shelf Sci. 2003, 56, 251–259. [Google Scholar] [CrossRef]
- Huxham, M.; Kumara, M.P.; Jayatissa, L.P.; Krauss, K.W.; Kairo, J.; Langat, J.; Mencuccini, M.; Skov, M.W.; Kirui, B. Intra- and interspecific facilitation in mangroves may increase resilience to climate change threats. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2127–2135. [Google Scholar] [CrossRef]
- Kumara, M.P.; Jayatissa, L.P.; Krauss, K.W.; Phillips, D.H.; Huxham, M. High mangrove density enhances surface accretion, surface elevation change, and tree survival in coastal areas susceptible to sea-level rise. Oecologia 2010, 164, 545–553. [Google Scholar] [CrossRef]
- Cahoon, D.R.; McKee, K.L.; Morris, J.T. How plants influence resilience of salt marsh and mangrove wetlands to sea-level rise. Estuaries Coasts 2020, 44, 883–898. [Google Scholar] [CrossRef]
- Mazda, Y.; Magi, M.; Ikeda, Y.; Kurokawa, T.; Asano, T. Wave reduction in a mangrove forest dominated by Sonneratia sp. Wetl. Ecol. Manag. 2006, 14, 365–378. [Google Scholar] [CrossRef]
- Spenceley, A. The role of pneumatophores in sedimentary processes. Mar. Geol. 1977, 24, M31–M37. [Google Scholar] [CrossRef]
- Karimi, Z.; Abdi, E.; Deljouei, A.; Cislaghi, A.; Shirvany, A.; Schwarz, M.; Hales, T.C. Vegetation-induced soil stabilization in coastal area: An example from a natural mangrove forest. Catena 2022, 216, 106410. [Google Scholar] [CrossRef]
- Cinco-Castro, S.; Herrera-Silveira, J.; Comín, F. Sedimentation as a support ecosystem service in different ecological types of mangroves. Front. For. Glob. Chang. 2022, 5, 733820. [Google Scholar] [CrossRef]
- Kathiresan, K. How do mangrove forests induce sedimentation? Rev. Biol. Trop. 2003, 51, 355–360. [Google Scholar]
- Al-Khayat, J.A.; Alatalo, J.M. Relationship between tree size, sediment mud content, oxygen levels, and pneumatophore abundance in the mangrove tree species Avicennia marina (Forssk.) Vierh. J. Mar. Sci. Eng. 2021, 9, 100. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Kairo, J.G.; Bondt, R.D.; Koedam, N. Pneumatophore height and density in relation to micro-topography in the grey mangrove Avicennia marina. Belg. J. Bot. 2007, 140, 213–221. [Google Scholar] [CrossRef]
- Kazemi, A.; Castillo, L.; Curet, O.M. Mangrove roots model suggest an optimal porosity to prevent erosion. Sci. Rep. 2021, 11, 9969–9982. [Google Scholar] [CrossRef]
- Mullarney, J.; Henderson, S.; Norris, B.; Bryan, K.; Fricke, A.; Sandwell, D.; Culling, D.A. A question of scale: How turbulence around aerial roots shapes the seabed morphology in mangrove forests of the Mekong delta. Oceanography 2017, 30, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Parr, J.; Pukotchasarnseen, T.; La-Orphanphol, T. Bang Pu: Thailand’s first urban nature education centre. Nat. Hist. Bull. Siam Soc. 2012, 58, 7–17. [Google Scholar]
- Balke, T.; Webb, E.L.; Elzen, E.V.D.; Galli, D.; Herman, P.M.J.; Bouma, T.J. Seedling establishment in a dynamic sedimentary environment: A conceptual framework using mangroves. J. Appl. Ecol. 2013, 50, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Round, P.D. Shorebirds in the Inner Gulf of Thailand. Stilt 1998, 50, 96–102. [Google Scholar]
- Komiyama, A.; Poungparn, S.; Kato, S. Common allometric equations for estimating the tree weight of mangroves. J. Trop. Ecol. 2005, 21, 471–477. [Google Scholar] [CrossRef]
- Ross, P.M. Macrofaunal Loss and Microhabitat Destruction: The impact of trampling in a temperate mangrove forest, NSW Australia. Wetl. Ecol. Manag. 2006, 14, 167–184. [Google Scholar] [CrossRef]
- Middleton, B.A.; McKee, K.L. Degradation of mangrove tissues and implications for peat formation in Belizean island forests. J. Ecol. 2001, 89, 818–828. [Google Scholar] [CrossRef]
- Adame, M.F.; Neil, D.T.; Wright, S.F.; Lovelock, C.E. Sedimentation within and among mangrove forests along a gradient of geomorphological settings. Estuar. Coast. Shelf Sci. 2010, 86, 21–30. [Google Scholar] [CrossRef]
- Marion, C.; Anthony, E.J.; Trentesaux, A. Short-term (≤2 yrs) estuarine mudflat and saltmarsh sedimentation: High-resolution data from ultrasonic altimetery, rod surface-elevation table, and filter traps. Estuar. Coast. Shelf Sci. 2009, 83, 475–484. [Google Scholar] [CrossRef]
- Samosorn, S.; Sangtiean, T.; Rodtassana, C.; Poungparn, S. Roles of aboveground roots facilitating sedimentation and elevation change in a mangrove forest behind bamboo seawalls. Songklanakarin J. Sci. Techno. 2018, 40, 1315–1323. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Estimation of colloidal materials in soils. Science 1926, 64, 362-362. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Jimenez, J.A.; Lugo, A.E.; Cintrón, G. Tree mortality in mangrove forests. Biotropica 1985, 17, 177. [Google Scholar] [CrossRef] [Green Version]
- Azman, M.S.; Sharma, S.; Shaharudin, M.A.M.; Hamzah, M.L.; Adibah, S.N.; Zakaria, R.M.; MacKenzie, R.A. Stand structure, biomass and dynamics of naturally regenerated and restored mangroves in Malaysia. For. Ecol. Manag. 2020, 482, 118852. [Google Scholar] [CrossRef]
- Deshar, R.; Sharma, S.; Hoque, A.; Mouctara, K.; Hagihara, A. Self-thinning of leaf, wood and aboveground in overcrowded mangrove Bruguiera gymnorrhiza stands in Okinawa Island, Japan. Procedia Environ. Sci. 2012, 13, 982–993. [Google Scholar] [CrossRef] [Green Version]
- McKee, K. Seedling recruitment patterns in a Belizean mangrove forest: Effects of establishment ability and physico-chemical factors. Oecologia 1995, 101, 448–460. [Google Scholar] [CrossRef]
- Osunkoya, O.; Creese, R.G. Population structure, spatial pattern and seedling establishment of the grey mangrove, Avicennia marina var. australasica, in New Zealand. Aust. J. Bot. 1997, 45, 707–725. [Google Scholar] [CrossRef]
- Balke, T.; Bouma, T.J.; Horstman, E.M.; Webb, E.L.; Erftemeijer, P.L.A.; Herman, P.M.J. Windows of opportunity: Thresholds to mangrove seedling establishment on tidal flats. Mar. Ecol. Prog. Ser. 2011, 440, 1–9. [Google Scholar] [CrossRef] [Green Version]
- McKee, K.L.; Rooth, J.E.; Feller, I. Mangrove recruitment after forest disturbance is facilitated by herbaceous species in the Caribbean. Ecol. Appl. 2007, 17, 1678–1693. [Google Scholar] [CrossRef]
- Sousa, W.P.; Kennedy, P.G.; Mitchell, B.J.; Ordóñez L, B.M. Supply-side ecology in mangroves: Do propagule dispersal and seedling establishment explain forest structure? Ecol. Monogr. 2007, 77, 53–76. [Google Scholar] [CrossRef]
- Rabinowitz, D. Early growth of mangrove seedlings in Panama, and an hypothesis concerning the relationship of dispersal and zonation. J. Biogeogr. 1978, 5, 113. [Google Scholar] [CrossRef]
- Toma, T.; Nakamura, K.; Patanaponpaiboon, P.; Ogino, K. Effect of flooding water level and plant density on growth of pneumatophore of Avicennia marina. Tropics 1991, 1, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.R.; Barba, E.; Choix, F.J. Production and biomass of mangrove roots in relation to hydroperiod and physico-chemical properties of sediment and water in the Mecoacan Lagoon, Gulf of Mexico. Wetl. Ecol. Manag. 2019, 27, 427–442. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, C. The vertical accretion of sediment under the alien mangrove species Sonneratia apetala and the indigenous species Aegiceras corniculatum. Wetl. Ecol. Manag. 2020, 28, 595–606. [Google Scholar] [CrossRef]
- Norris, B.K.; Mullarney, J.C.; Bryan, K.R.; Henderson, S.M. The effect of pneumatophore density on turbulence: A field study in a Sonneratia-dominated mangrove forest, Vietnam. Cont. Shelf Res. 2017, 147, 114–127. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Thompson, C.; Wang, X.; Cai, T.; Chang, Y. Differential sediment trapping abilities of mangrove and saltmarsh vegetation in a subtropical estuary. Geomorphology 2018, 318, 270–282. [Google Scholar] [CrossRef]
- Furukawa, K.; Wolanski, E. Sedimentation in Mangrove Forests. Mangroves Salt Marshes 1996, 1, 3–10. [Google Scholar] [CrossRef]
- Hashim, R.; Kamali, B.; Tamin, N.M.; Zakaria, R. An integrated approach to coastal rehabilitation: Mangrove restoration in Sungai Haji Dorani, Malaysia. Estuar. Coast. Shelf Sci. 2010, 86, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Han, S.H.; Kim, S.; An, J.; Alatalo, J.M.; Son, Y. Interactions between topsoil properties and ecophysiological responses of mangroves (Avicennia marina) along the tidal gradient in an arid region in Qatar. Turk. J. Agric. For. 2020, 44, 121–126. [Google Scholar] [CrossRef]
- Rasmeemasmuang, T.; Sasaki, J. Wave reduction in mangrove forests. In Handbook of Coastal Disaster Mitigation for Engineers and Planners; Esteban, M., Takagi, H., Shibayama, T., Eds.; Elsevier: Oxford, UK, 2015; pp. 511–535. [Google Scholar]
- Van Santen, P.; Augustinus, P.; Janssen-Stelder, B.; Quartel, S.; Tri, N. Sedimentation in an estuarine mangrove system. J. Southeast Asian Earth Sci. 2007, 29, 566–575. [Google Scholar] [CrossRef]
- Furukawa, K.; Wolanski, E.; Mueller, H. Currents and sediment transport in mangrove forests. Estuar. Coast. Shelf Sci. 1997, 44, 301–310. [Google Scholar] [CrossRef]
- Tue, N.T.; Ngoc, N.T.; Quy, T.D.; Hamaoka, H.; Nhuan, M.T.; Omori, K. A cross-system analysis of sedimentary organic carbon in the mangrove ecosystems of Xuan Thuy National Park, Vietnam. J. Sea Res. 2012, 67, 69–76. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Kuzyakov, Y.; Lubis, A.A.; Murdiyarso, D.; Hutley, L.B.; Bachri, S.; Friess, D.A.; Martius, C.; Borchard, N. Organic carbon burial and sources in soils of coastal mudflat and mangrove ecosystems. Catena 2020, 187, 104414. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Liao, B.; Huang, B.; Wu, F.; Jiang, Z. The effect of vegetation on surface elevation in coastal mangrove areas. J. Coast. Res. 2020, 36, 600. [Google Scholar] [CrossRef]
- Krauss, K.W.; Cahoon, D.R.; Allen, J.A.; Ewel, K.C.; Lynch, J.C.; Cormier, N. Surface elevation change and susceptibility of different mangrove zones to sea-level rise on pacific high islands of Micronesia. Ecosystems 2010, 13, 129–143. [Google Scholar] [CrossRef]
- Thampanya, U.; Vermaat, J.; Duarte, C.M. Colonization success of common Thai mangrove species as a function of shelter from water movement. Mar. Ecol. Prog. Ser. 2002, 237, 111–120. [Google Scholar] [CrossRef]
- Du, Q.; Qin, Z.; Ming, S.; Zhang, C. Differences in the vertical accretion of sediment among mangrove species with different aerial root types. Estuar. Coast. Shelf Sci. 2021, 256, 107375–107386. [Google Scholar] [CrossRef]







| Distance from the Shore (m) | Tree Density (Trees 40 m−2) | Total Basal Area (m2 40 m−2) | Biomass (kg 40 m−2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aboveground | Belowground | Total | ||||||||
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| 0−10 | 6 ± 0.9 | 5 ± 0.8 | 0.0704 | 0.0843 | 411 | 533 | 178 | 221 | 589 | 754 |
| 10−20 | 5 ± 1.3 | 5 ± 1.4 | 0.0602 | 0.0619 | 348 | 371 | 152 | 159 | 500 | 530 |
| 20−30 | 5 ± 1.2 | 4 ± 1.0 | 0.0836 | 0.0910 | 510 | 579 | 216 | 240 | 726 | 819 |
| 30−40 | 4 ± 1.0 | 3 ± 1.0 | 0.0766 | 0.0820 | 471 | 517 | 199 | 216 | 670 | 733 |
| 40−50 | 5 ± 1.0 | 5 ± 1.0 | 0.0809 | 0.0864 | 487 | 530 | 208 | 224 | 695 | 753 |
| 50−60 | 5 ± 1.3 | 5 ± 1.3 | 0.0880 | 0.0966 | 519 | 581 | 224 | 248 | 743 | 829 |
| 60−70 | 6 ± 1.0 | 5 ± 1.1 | 0.1207 | 0.1129 | 761 | 721 | 317 | 298 | 1078 | 1019 |
| 70−80 | 5 ± 1.3 | 5 ± 1.3 | 0.0725 | 0.0770 | 407 | 438 | 180 | 193 | 587 | 631 |
| 80−90 | 6 ± 1.1 | 5 ± 1.0 | 0.0932 | 0.0906 | 539 | 533 | 235 | 230 | 775 | 764 |
| 90−100 | 7 ± 0.5 | 7 ± 0.6 | 0.0983 | 0.1007 | 562 | 581 | 246 | 253 | 809 | 835 |
| Year | Soil Parameters | Depth (cm) | Distance (m) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mudflat | 0 | 10 | 30 | 50 | 70 | 90 | |||
| 2020 | Bulk Density (g cm−3) | 0−10 | 1.39 ± 0.05 ns | 1.21 ± 0.12 ns | 1.39 ± 0.07 ns | 1.40 ± 0.07 ns | 1.37 ± 0.07 ns | 1.30 ± 0.06 ns | 1.33 ± 0.08 ns |
| 0−20 | 1.09 ± 0.07 bc | 0.98 ± 0.07 c | 1.22 ± 0.08 ab | 1.33 ± 0.04 a | 1.33 ± 0.03 a | 1.32 ± 0.06 a | 1.26 ± 0.06 ab | ||
| Sand (%) | 0−20 | 0 bc | 6.43 abc | 10.88 a | 7.29 ab | 1.70 abc | 2.03 bc | 0 c | |
| Silt (%) | 89.88 ns | 82.60 ns | 82.59 ns | 86.09 ns | 90.35 ns | 90.29 ns | 89.96 ns | ||
| Clay (%) | 10.12 ns | 10.96 ns | 6.53 ns | 6.67 ns | 7.98 ns | 7.67 ns | 10.04 ns | ||
| Soil texture | Silt | Silty loam | Silty loam | Silty loam | Silt | Silt | Silt | ||
| Organic matter (%) | 1.34 ± 0.14 ac | 2.09 ± 0.23 b | 1.26 ± 0.18 cd | 1.09 ± 0.08 de | 0.94 ± 0.09 e | 1.19 ± 0.06 cd | 1.51 ± 0.11 ab | ||
| 2021 | Bulk density (g cm−3) | 0−10 | 1.26 ± 0.06 ABC | 1.17 ± 0.05 C | 1.33 ± 0.06 ABC | 1.43 ± 0.13 AB | 1.44 ± 0.07 A | 1.43 ± 0.08 A | 1.19 ± 0.03 BC |
| 0−20 | 1.22 ± 0.06 AB | 1.03 ± 0.07 B | 1.08 ± 0.02 B | 1.48 ± 0.08 A | 1.23 ± 0.09 AB | 1.31 ± 0.08 A | 1.19 ± 0.09 AB | ||
| Sand (%) | 0−20 | 1.95 B | 6.84 AB | 13.32 A | 11.92 A | 8.35 AB | 8.02 AB | 3.24 B | |
| Silt (%) | 84.27 ns | 78.69 ns | 78.04 ns | 80.93 ns | 84.39 ns | 83.87 ns | 83.92 ns | ||
| Clay (%) | 13.79 AB | 14.47 AB | 8.64 BC | 7.25 C | 7.26 C | 8.11 BC | 12.84 A | ||
| Soil texture | Silty loam | Silty loam | Silty loam | Silt | Silt | Silt | Silt | ||
| Organic matter (%) | 1.56 ± 0.24 ABC | 2.38 ± 0.53 A | 1.42 ± 0.15 BC | 1.22 ± 0.05 CD | 1.04 ± 0.08 D | 1.29 ± 0.15 CD | 1.77 ± 0.17 AB | ||
| Variable | r/rho | p-Value |
|---|---|---|
| Distance from the shore with: | ||
| Total BA of trees | 0.194 | 0.035 |
| Seedling density | 0.739 | <0.001 |
| Hpneumatophore | −0.817 | <0.001 |
| BApneumatophore | −0.587 | <0.001 |
| SApneumatophore | −0.728 | <0.001 |
| Vpneumatophore | −0.786 | <0.001 |
| Fine root density | 0.515 | 0.001 |
| Inundation period with: | ||
| Relative elevation | −0.631 | <0.001 |
| D0 pneumatophore | 0.341 | 0.042 |
| Hpneumatophore | 0.765 | <0.001 |
| BApneumatophore | 0.569 | <0.001 |
| SApneumatophore | 0.660 | <0.001 |
| Vpneumatophore | 0.748 | <0.001 |
| Seedling density with: | ||
| BApneumatophore | −0.346 | 0.039 |
| Vpneumatophore | −0.578 | <0.001 |
| Fine root density | 0.605 | <0.001 |
| Sedimentation rate with: | ||
| Hpneumatophore | −0.211 | 0.011 |
| SApneumatophore | −0.193 | 0.021 |
| Vpneumatophore | −0.206 | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hongwiset, S.; Rodtassana, C.; Poungparn, S.; Umnouysin, S.; Suchewaboripont, V. Synergetic Roles of Mangrove Vegetation on Sediment Accretion in Coastal Mangrove Plantations in Central Thailand. Forests 2022, 13, 1739. https://doi.org/10.3390/f13101739
Hongwiset S, Rodtassana C, Poungparn S, Umnouysin S, Suchewaboripont V. Synergetic Roles of Mangrove Vegetation on Sediment Accretion in Coastal Mangrove Plantations in Central Thailand. Forests. 2022; 13(10):1739. https://doi.org/10.3390/f13101739
Chicago/Turabian StyleHongwiset, Sarawan, Chadtip Rodtassana, Sasitorn Poungparn, Suthathip Umnouysin, and Vilanee Suchewaboripont. 2022. "Synergetic Roles of Mangrove Vegetation on Sediment Accretion in Coastal Mangrove Plantations in Central Thailand" Forests 13, no. 10: 1739. https://doi.org/10.3390/f13101739
APA StyleHongwiset, S., Rodtassana, C., Poungparn, S., Umnouysin, S., & Suchewaboripont, V. (2022). Synergetic Roles of Mangrove Vegetation on Sediment Accretion in Coastal Mangrove Plantations in Central Thailand. Forests, 13(10), 1739. https://doi.org/10.3390/f13101739