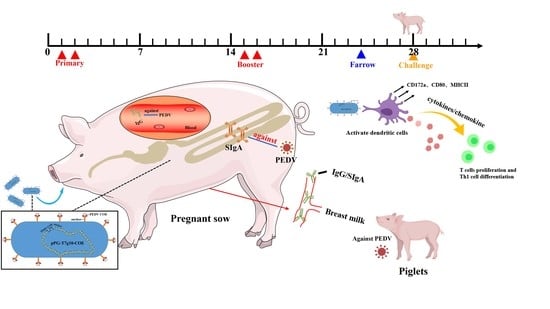
Immune Responses in Pregnant Sows Induced by Recombinant Lactobacillus johnsonii Expressing the COE Protein of Porcine Epidemic Diarrhea Virus Provide Protection for Piglets against PEDV Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterium, Plasmid and Virus
2.2. Construction of Recombinant Lactobacillus johnsonii
2.3. Protein Expression by Recombinant Lactobacillus johnsonii
2.4. Isolation and Culture of Porcine Monocyte-Derived Dendritic Cells (MoDCs)
2.5. Scanning Electron Microscopy (SEM) Observation
2.6. Detection of MoDCs Surface Markers
2.7. Relative Expression Analysis of Cytokines
2.8. L. johnsonii-COE Activated MoDCs Induced CD4+ T Cell Proliferation and Differentiation
2.9. Oral Immunization of Pregnant Sows
2.10. Analysis of Antibody Levels by ELISA
2.11. Analysis of Antibody Levels in Piglets
2.12. Challenge, PEDV Fecal Shedding Analysis and Clinical Evaluation
2.13. Histopathological Analysis
2.14. Immunohistochemistry
2.15. Statistical Analysis
3. Results
3.1. Analysis of COE Expression in L. johnsonii
3.2. Morphological Observation of Porcine MoDCs and L. johnsonii-COE Adherence to MoDCs
3.3. L. johnsonii-COE Activated the Maturation of the MoDCs by Flow Cytometry
3.4. MoDCs Activated by L. johnsonii-COE Promoted CD4+ T Cell Proliferation
3.5. L. johnsonii-COE Activated MoDCs Expressed Cytokines
3.6. MoDCs Activated by L. johnsonii-COE Have Ability to Drive CD4+ T Cell toward Th1 Subset Polarization
3.7. PEDV-Specific Antibody Levels in Sows Induced by L. johnsonii-COE
3.8. Cytokines Detection in the Serum of Pregnant Sows
3.9. Antibody Levels in the Colostrum
3.10. Detection of Specific Anti-PEDV SIgA and IgG Antibodies in Piglets
3.11. The Detection of Fecal Viral Shedding in Piglets and Clinical Symptoms Evaluation
3.12. Histopathological Observation and Immunohistochemistry Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Jiang, X.; Jiang, Y.; Tang, L.; Xu, Y.; Qiao, X.; Min, L.; Wen, C.; Ma, G.; Li, Y. Oral immunization against PEDV with recombinant Lactobacillus casei expressing dendritic cell-targeting peptide fusing COE protein of PEDV in piglets. Viruses 2018, 10, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, Y.; Tian, Y.; Li, Y.; Xue, R.; Hu, L.; Zhang, D.; Sun, S.; Wang, G.; Chen, J.; Lan, Z.; et al. Recombinant Lactobacillus acidophilus expressing S1 and S2 domains of porcine epidemic diarrhea virus could improve the humoral and mucosal immune levels in mice and sows inoculated orally. Vet. Microbiol. 2020, 248, 108827. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Huang, X.; Ma, S.; Yu, M.; Shi, W.; Qiao, X.; Tang, L.; Xu, Y.; Li, Y. Oral delivery of probiotics expressing dendritic cell-targeting peptide fused with porcine epidemic diarrhea virus COE antigen: A promising vaccine strategy against PEDV. Viruses 2017, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Song, D.S.; Oh, J.S.; Kang, B.K.; Yang, J.S.; Moon, H.J.; Yoo, H.S.; Jang, Y.S.; Park, B.K. Oral efficacy of vero cell attenuated porcine epidemic diarrhea virus DR13 strain. Res. Vet. Sci. 2007, 82, 134–140. [Google Scholar] [CrossRef]
- Won, H.; Lee, D.U.; Jang, G.; Noh, Y.H.; Lee, S.C.; Choi, H.W.; Yoon, I.J.; Yoo, H.S.; Lee, C. Generation and protective efficacy of a cold-adapted attenuated genotype 2b porcine epidemic diarrhea virus. J. Vet. Sci. 2019, 20, e32. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Xu, Z.; Zhou, Q.; Li, W.; Wu, Y.; Du, Y.; Chen, L.; Xue, C.; Cao, Y. A heterologous ‘prime-boost’ anti-PEDV immunization for pregnant sows protects neonatal piglets through lactogenic immunity against PEDV. Lett. Appl. Microbiol. 2019, 69, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Y.; Yuan, W.; Peng, Q.; Zhang, F.; Ye, Y.; Huang, D.; Ding, Z.; Lin, L.; He, H.; et al. Evaluation of cross-protection between G1a- and G2a-genotype porcine epidemic diarrhea viruses in suckling piglets. Animals 2020, 10, 1674. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, P.H.; Leer, R.J.; Shaw, M.; Heijne den Bak-Glashouwer, M.J.; Tielen, F.D.; Smit, E.; Martinez, B.; Jore, J.; Conway, P.L. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int. J. Food Microbiol. 1998, 41, 155–167. [Google Scholar] [CrossRef]
- Scheppler, L.; Vogel, M.; Zuercher, A.W.; Zuercher, M.; Germond, J.E.; Miescher, S.M.; Stadler, B.M. Recombinant Lactobacillus johnsonii as a mucosal vaccine delivery vehicle. Vaccine 2002, 20, 2913–2920. [Google Scholar] [CrossRef]
- Scheppler, L.; Vogel, M.; Marti, P.; Muller, L.; Miescher, S.M.; Stadler, B.M. Intranasal immunisation using recombinant Lactobacillus johnsonii as a new strategy to prevent allergic disease. Vaccine 2005, 23, 1126–1134. [Google Scholar] [CrossRef]
- Jia, S.; Huang, X.; Li, H.; Zheng, D.; Wang, L.; Qiao, X.; Jiang, Y.; Cui, W.; Tang, L.; Li, Y.; et al. Immunogenicity evaluation of recombinant Lactobacillus casei W56 expressing bovine viral diarrhea virus E2 protein in conjunction with cholera toxin B subunit as an adjuvant. Microb. Cell Factories 2020, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, B.; Niu, C.; Jia, S.; Sun, C.; Wang, Z.; Jiang, Y.; Cui, W.; Wang, L.; Xu, Y. Dendritic cell targeting of bovine viral diarrhea virus E2 protein expressed by Lactobacillus casei effectively induces antigen-specific immune responses via oral vaccination. Viruses 2019, 11, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Wang, L.; Huang, X.; Wang, X.; Chen, S.; Shi, W.; Qiao, X.; Jiang, Y.; Tang, L.; Xu, Y.; et al. Oral recombinant lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb. Cell Factories 2018, 17, 20. [Google Scholar] [CrossRef]
- Summerfield, A.; Guzylack-Piriou, L.; Schaub, A.; Carrasco, C.; Tache, V.; Charley, B.; McCullough, K. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology 2003, 110, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Zheng, D.; Chen, S.; Shi, W.; Qiao, X.; Jiang, Y.; Tang, L.; Xu, Y.; Li, Y. Oral immunization with a Lactobacillus casei-based anti-porcine epidemic diarrhoea virus (PEDV) vaccine expressing microfold cell-targeting peptide Co1 fused with the COE antigen of PEDV. J. Appl. Microbiol. 2018, 124, 368–378. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, Z.; Sui, L.; Xu, Y.; Wang, L.; Qiao, X.; Cui, W.; Jiang, Y.; Zhou, H.; Tang, L.; et al. Lactobacillus johnsonii activates porcine monocyte derived dendritic cells maturation to modulate Th cellular immune response. Cytokine 2021, 144, 155581. [Google Scholar] [CrossRef]
- Ma, S.; Qiao, X.; Xu, Y.; Wang, L.; Zhou, H.; Jiang, Y.; Cui, W.; Huang, X.; Wang, X.; Tang, L.; et al. Screening and identification of a chicken dendritic cell binding peptide by using a phage display library. Front. Immunol. 2019, 6, 1853. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Gong, L.; Peng, P.; Liu, Y.; Xue, C.; Cao, Y. Porcine enteric alphacoronavirus inhibits IFN- α, IFN- β, OAS, Mx1, and PKR mRNA expression in infected Peyer’s patches in vivo. Front. Vet. Sci. 2020, 3, 449. [Google Scholar] [CrossRef] [PubMed]
- Rolinec, M.; Medo, J.; Gabor, M.; Miluchova, M.; Biro, D.; Simko, M.; Juracek, M.; Hanusovsky, O.; Schubertova, Z.; Galik, B. The effect of coconut oil addition to feed of pigs on rectal microbial diversity and bacterial abundance. Animals 2020, 10, 1764. [Google Scholar] [CrossRef]
- Song, L.; Xie, W.; Liu, Z.; Guo, D.; Zhao, D.; Qiao, X.; Wang, L.; Zhou, H.; Cui, W.; Jiang, Y.; et al. Oral delivery of a Lactococcus lactis strain secreting bovine lactoferricin-lactoferrampin alleviates the development of acute colitis in mice. Appl. Microbiol. Biotechnol. 2019, 103, 6169–6186. [Google Scholar] [CrossRef]
- Van der Wel, N.N.; Sugita, M.; Fluitsma, D.M.; Cao, X.; Schreibelt, G.; Brenner, M.B.; Peters, P.J. CD1 and major histocompatibility complex II molecules follow a different course during dendritic cell maturation. Mol. Biol. Cell 2003, 14, 3378–3388. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, Y.; Yang, Q. Transferrin receptor 1 levels at the cell surface influence the susceptibility of newborn piglets to PEDV infection. PLoS Pathog. 2020, 16, e1008682. [Google Scholar] [CrossRef]
- Gao, X.; Ma, Y.; Wang, Z.; Bai, J.; Jia, S.; Feng, B.; Jiang, Y.; Cui, W.; Tang, L.; Li, Y.; et al. Oral immunization of mice with a probiotic Lactobacillus casei constitutively expressing the alpha-toxoid induces protective immunity against Clostridium perfringens alpha-toxin. Virulence 2019, 10, 166–179. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, G.; Wang, J.; Man, K.; Yang, Q. Cell attenuated porcine epidemic diarrhea virus strain Zhejiang08 provides effective immune protection attributed to dendritic cell stimulation. Vaccine 2017, 35, 7033–7041. [Google Scholar] [CrossRef]
- Kapsenberg, M.L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003, 3, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Sierro, F.; Dubois, B.; Coste, A.; Kaiserlian, D.; Kraehenbuhl, J.P.; Sirard, J.C. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. USA 2001, 98, 13722–13727. [Google Scholar] [CrossRef] [Green Version]
- Maynard, C.L.; Weaver, C.T. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol. Rev. 2008, 226, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Ma, S.; Wang, L.; Zhou, H.; Jiang, Y.; Cui, W.; Qiao, X.; Xu, Y.; Li, Y.; Tang, L. Lactobacillus johnsonii-activated chicken bone marrow-derived dendritic cells exhibit maturation and increased expression of cytokines and chemokines in vitro. Cytokine 2020, 136, 155269. [Google Scholar] [CrossRef]
- Ghavami, S.B.; Yadegar, A.; Aghdaei, H.A.; Sorrentino, D.; Farmani, M.; Mir, A.S.; Azimirad, M.; Balaii, H.; Shahrokh, S.; Zali, M.R. Immunomodulation and generation of tolerogenic dendritic cells by probiotic bacteria in patients with inflammatory bowel disease. Int. J. Mol. Sci. 2020, 21, 6266. [Google Scholar] [CrossRef]
- Wan, Y.Y. Multi-tasking of helper T cells. Immunology 2010, 130, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Stone, S.; Drebes, D.; Greiner, L.; Dvorak, C.; Murtaugh, M. Characterization of anti-porcine epidemic diarrhea virus neutralizing activity in mammary secretions. Virus Res. 2016, 226, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coombes, J.L.; Powrie, F. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 2008, 8, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Indrelid, S.; Kleiveland, C.; Holst, R.; Jacobsen, M.; Lea, T. The soil bacterium Methylococcus capsulatus Bath interacts with human dendritic cells to modulate immune function. Front. Microbiol. 2017, 8, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Primer Sequence (5′-3′) | Accession Number | |
|---|---|---|
| β-actin | F- GGTGGGTATGGGTCAGAAAG | AF054837 |
| R- TCCATGTCGTCCCAGTTGGT | ||
| IL10 | F- GGAAGACGTAATGCCGAAGG | NM_214041 |
| R- GGCACTCTTCACCTCCTCCA | ||
| IL12p40 | F- TGGACCTCAGACCAGAGCAG | U08317 |
| R- GCAGGAGTGACTGGCTCAGA | ||
| CCL-20 | F- TGCTCCTGGCTGCTTTGATGTC | AJ577084.1 |
| R- TCATTGGCGAGCTGCTGTGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Wang, X.; Ju, N.; Wang, Z.; Sui, L.; Wang, L.; Qiao, X.; Cui, W.; Jiang, Y.; Zhou, H.; et al. Immune Responses in Pregnant Sows Induced by Recombinant Lactobacillus johnsonii Expressing the COE Protein of Porcine Epidemic Diarrhea Virus Provide Protection for Piglets against PEDV Infection. Viruses 2022, 14, 7. https://doi.org/10.3390/v14010007
Zheng D, Wang X, Ju N, Wang Z, Sui L, Wang L, Qiao X, Cui W, Jiang Y, Zhou H, et al. Immune Responses in Pregnant Sows Induced by Recombinant Lactobacillus johnsonii Expressing the COE Protein of Porcine Epidemic Diarrhea Virus Provide Protection for Piglets against PEDV Infection. Viruses. 2022; 14(1):7. https://doi.org/10.3390/v14010007
Chicago/Turabian StyleZheng, Dianzhong, Xiaona Wang, Ning Ju, Zhaorui Wang, Ling Sui, Li Wang, Xinyuan Qiao, Wen Cui, Yanping Jiang, Han Zhou, and et al. 2022. "Immune Responses in Pregnant Sows Induced by Recombinant Lactobacillus johnsonii Expressing the COE Protein of Porcine Epidemic Diarrhea Virus Provide Protection for Piglets against PEDV Infection" Viruses 14, no. 1: 7. https://doi.org/10.3390/v14010007
APA StyleZheng, D., Wang, X., Ju, N., Wang, Z., Sui, L., Wang, L., Qiao, X., Cui, W., Jiang, Y., Zhou, H., Li, Y., & Tang, L. (2022). Immune Responses in Pregnant Sows Induced by Recombinant Lactobacillus johnsonii Expressing the COE Protein of Porcine Epidemic Diarrhea Virus Provide Protection for Piglets against PEDV Infection. Viruses, 14(1), 7. https://doi.org/10.3390/v14010007