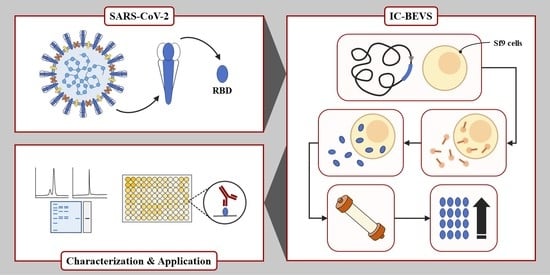
Improved Expression of SARS-CoV-2 Spike RBD Using the Insect Cell-Baculovirus System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of pFBD-polh-pSeL-X Baculovirus Shuttle Vector
2.2. RBD cDNA Sequence and Cloning into pFBD-polh-pSeL-X Baculovirus Shuttle Vector
2.3. Insect Cell Culture
2.4. Virus Production
2.5. Insect Cell Infection
2.6. Total Protein Measurement
2.7. Electrophoretic Analysis
2.8. rRBD Purification by Immobilized Metal Ion Affinity Chromatography (IMAC)
2.9. MALDI-TOF Mass Spectrometry
2.10. Glycosylation Assay
2.11. Reverse-Phase-High Performance Liquid Chromatography (RP-HPLC)
2.12. Size Exclusion Chromatography (SEC)
2.13. Assessment of rRBD Immunoreactivity
2.13.1. Serum Collection
2.13.2. Bridge Enzyme-Linked Immunosorbent Assay (b-ELISA) Using rRBD as Coating Antigen
3. Results and Discussion
3.1. Generation of the Recombinant Baculovirus Acpolh-pSeL-gprRBD
3.2. rRBD Expression in Sf9 Insect Cells
3.3. Purification of rRBD from Sf9 Cell Line
3.4. Characterization of the Purified RBD from Sf9 Insect Cell Culture
3.5. Use of the RBD to Detect Anti-SARS-CoV-2 IgG in Serum Samples from COVID-19-Positive Patients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Wu, C.-R.; Yin, W.-C.; Jiang, Y.; Xu, H.E. Structure Genomics of SARS-CoV-2 and Its Omicron Variant: Drug Design Templates for COVID-19. Acta Pharmacol. Sin. 2022, 43, 3021–3033. [Google Scholar] [CrossRef] [PubMed]
- Abduljaleel, Z.; Shahzad, N.; Aziz, S.A.; Malik, S.M. Monoclonal Antibody Designed for SARS-NCoV-2 Spike Protein of Receptor Binding Domain on Antigenic Targeted Epitopes for Inhibition to Prevent Viral Entry. Mol. Divers. 2022, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, D.; Han, Y.; Lu, M. Structural Plasticity and Immune Evasion of SARS-CoV-2 Spike Variants. Viruses 2022, 14, 1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, C.; Zhang, H.; Cao, G.; Wang, S.; Yin, S.; Wang, Y. SARS-CoV-2 Tetrameric RBD Protein Blocks Viral Infection and Induces Potent Neutralizing Antibody Response. Front. Immunol. 2022, 13, 960094. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A Vaccine Targeting the RBD of the S Protein of SARS-CoV-2 Induces Protective Immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- Smith, I.; Mc Callum, G.J.; Sabljic, A.V.; Marfia, J.I.; Bombicino, S.S.; Trabucchi, A.; Iacono, R.F.; Birenbaum, J.M.; Vazquez, S.C.; Minoia, J.M.; et al. Rapid and Cost-Effective Process Based on Insect Larvae for Scale-up Production of SARS-COV-2 Spike Protein for Serological COVID-19 Testing. Biotechnol. Bioeng. 2021, 118, 4129–4137. [Google Scholar] [CrossRef]
- Fan, Y.; Wei, J.; Wang, W.; Li, C.; Pan, G.; Keiffer, T.; Bao, J.; Zhou, Z. Utilization of Recombinant Baculovirus Expression System to Produce the RBD Domain of SARS-CoV-2 Spike Protein. Pathogens 2022, 11, 672. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron Escapes the Majority of Existing SARS-CoV-2 Neutralizing Antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, N.; Halder, A. Preventive diagnostic and therapeutic application of baculovirus expression vector system. In Trends in Insect Molecular Biololy and Biotechnology; Kumar, D., Gong, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 163–191. [Google Scholar] [CrossRef]
- Targovnik, A.M.; Simonin, J.A.; Mc Callum, G.J.; Smith, I.; Cuccovia Warlet, F.U.; Nugnes, M.V.; Miranda, M.V.; Belaich, M.N. Solutions against Emerging Infectious and Noninfectious Human Diseases through the Application of Baculovirus Technologies. Appl. Microbiol. Biotechnol. 2021, 105, 8195–8226. [Google Scholar] [CrossRef]
- Azali, M.A.; Mohamed, S.; Harun, A.; Hussain, F.A.; Shamsuddin, S.; Johan, M.F. Application of Baculovirus Expression Vector System (BEV) for COVID-19 Diagnostics and Therapeutics: A Review. J. Genet. Eng. Biotechnol. 2022, 20, 98. [Google Scholar] [CrossRef]
- Ikonomou, L.; Schneider, Y.J.; Agathos, S.N. Insect Cell Culture for Industrial Production of Recombinant Proteins. Appl. Microbiol. Biotechnol. 2003, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, T.; Xue, W.; Zhang, S.; Cui, L.; Wang, H.; Zhang, Y.; Zhou, L.; Gu, Y.; Xia, N.; et al. Genetic Engineering of Baculovirus-Insect Cell System to Improve Protein Production. Front. Bioeng. Biotechnol. 2022, 10, 1–15. [Google Scholar] [CrossRef]
- Klausberger, M.; Duerkop, M.; Haslacher, H.; Wozniak-Knopp, G.; Cserjan-Puschmann, M.; Perkmann, T.; Lingg, N.; Aguilar, P.P.; Laurent, E.; De Vos, J.; et al. A Comprehensive Antigen Production and Characterisation Study for Easy-to-Implement, Specific and Quantitative SARS-CoV-2 Serotests. EBioMedicine 2021, 67, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Montemiglio, L.C.; Vitagliano, G.; Fedele, L.; Sellathurai, S.; Bucci, F.; Compagnone, M.; Chiarini, V.; Exertier, C.; Muzi, A.; et al. The Nuts and Bolts of SARS-CoV-2 Spike Receptor-Binding Domain Heterologous Expression. Biomolecules 2021, 11, 1812. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A Serological Assay to Detect SARS-CoV-2 Seroconversion in Humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Solís, M.; Herrero, S.; Targovnik, A.M. Engineering of the Baculovirus Expression System for Optimized Protein Production. Appl. Microbiol. Biotechnol. 2019, 103, 113–123. [Google Scholar] [CrossRef]
- Choque-Guevara, R.; Poma-Acevedo, A.; Montesinos-Millán, R.; Rios-Matos, D.; Gutiérrez-Manchay, K.; Montalvan-Avalos, A.; Quiñones-Garcia, S.; de Grecia Cauti-Mendoza, M.; Agurto-Arteaga, A.; Ramirez-Ortiz, I.; et al. Squalene in Oil-Based Adjuvant Improves the Immunogenicity of SARS-CoV-2 RBD and Confirms Safety in Animal Models. PLoS ONE 2022, 17, e0269823. [Google Scholar] [CrossRef]
- Martínez-Solís, M.; Gómez-Sebastián, S.; Escribano, J.M.; Jakubowska, A.K.; Herrero, S. A Novel Baculovirus-Derived Promoter with High Activity in the Baculovirus Expression System. PeerJ 2016, 4, e2183. [Google Scholar] [CrossRef] [Green Version]
- Targovnik, A.M.; Ferrari, A.; Mc Callum, G.J.; Arregui, M.B.; Smith, I.; Bracco, L.F.; Alfonso, V.; López, M.G.; Martínez-Solís, M.; Herrero, S.; et al. Highly Efficient Production of Rabies Virus Glycoprotein G Ectodomain in Sf9 Insect Cells. 3 Biotech 2019, 9, 385. [Google Scholar] [CrossRef]
- O′Reilly, D.; Miller, L.; Luckow, V. Baculovirus Expression Vector: A Laboratory Manual; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Targovnik, A.M.; Romero, L.V.; Wolman, F.J.; Cascone, O.; Miranda, M.V. Horseradish Peroxidase Production from Spodoptera Frugiperda Larvae: A Simple and Inexpensive Method. Process Biochem. 2010, 45, 835–840. [Google Scholar] [CrossRef]
- Bradford A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizin the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [CrossRef]
- Trabucchi, A.; Bombicino, S.S.; Marfía, J.I.; Sabljic, A.V.; Iacono, R.F.; Smith, I.; Mc callum, G.J.; Targovnik, A.M.; Wolman, F.J.; Fingermann, M.; et al. Novel Bridge Multi-Species ELISA for Detection of SARS-CoV-2 Antibodies. J. Immunol. Methods 2022, 511, 113365. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-J.; König-Beihammer, J.; Vavra, U.; Schwestka, J.; Kienzl, N.F.; Klausberger, M.; Laurent, E.; Grünwald-Gruber, C.; Vierlinger, K.; Hofner, M.; et al. N-Glycosylation of the SARS-CoV-2 Receptor Binding Domain Is Important for Functional Expression in Plants. Front. Plant Sci. 2021, 12, 1154. [Google Scholar] [CrossRef] [PubMed]
- Mabashi-Asazuma, H.; Kuo, C.W.; Khoo, K.H.; Jarvis, D.L. A Novel Baculovirus Vector for the Production of Nonfucosylated Recombinant Glycoproteins in Insect Cells. Glycobiology 2014, 24, 325–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomares, L.A.; Srivastava, I.K.; Ramírez, O.T.; Cox, M.M.J. Glycobiotechnology of the Insect Cell-Baculovirus Expression System Technology. Adv. Biochem. Eng. Biotechnol. 2021, 175, 71–92. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Dalvie, N.C.; Rodriguez-Aponte, S.A.; Hartwell, B.L.; Tostanoski, L.H.; Biedermann, A.M.; Crowell, L.E.; Kaur, K.; Kumru, O.S.; Carter, L.; Yu, J.; et al. Engineered SARS-CoV-2 Receptor Binding Domain Improves Manufacturability in Yeast andImmunogenicity in Mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2106845118. [Google Scholar] [CrossRef]
- Klausberger, M.; Kienzl, N.F.; Stadlmayr, G.; Grünwald-Gruber, C.; Laurent, E.; Stadlbaer, K.; Stracke, F.; Vierlinger, K.; Hofner, M.; Manhart, G.; et al. Designed SARS-CoV-2 Receptor Binding Domain Variants Form Stable Monomers. Biotechnol. J. 2022, 17, 2100422. [Google Scholar] [CrossRef]
- An, Y.; Li, S.; Jin, X.; Han, J.B.; Xu, K.; Xu, S.; Han, Y.; Liu, C.; Zheng, T.; Liu, M.; et al. A Tandem-Repeat Dimeric RBD Protein-Based Covid-19 Vaccine Zf2001 Protects Mice and Nonhuman Primates. Emerg. Microbes Infect. 2022, 11, 1058–1071. [Google Scholar] [CrossRef]
- Pan, X.; Shi, J.; Hu, X.; Wu, Y.; Zeng, L.; Yao, Y.; Shang, W.; Liu, K.; Gao, G.; Guo, W.; et al. RBD-Homodimer, a COVID-19 Subunit Vaccine Candidate, Elicits Immunogenicity and Protection in Rodents and Nonhuman Primates. Cell Discov. 2021, 7, 82. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poodts, J.; Smith, I.; Birenbaum, J.M.; Rodriguez, M.S.; Montero, L.; Wolman, F.J.; Marfía, J.I.; Valdez, S.N.; Alonso, L.G.; Targovnik, A.M.; et al. Improved Expression of SARS-CoV-2 Spike RBD Using the Insect Cell-Baculovirus System. Viruses 2022, 14, 2794. https://doi.org/10.3390/v14122794
Poodts J, Smith I, Birenbaum JM, Rodriguez MS, Montero L, Wolman FJ, Marfía JI, Valdez SN, Alonso LG, Targovnik AM, et al. Improved Expression of SARS-CoV-2 Spike RBD Using the Insect Cell-Baculovirus System. Viruses. 2022; 14(12):2794. https://doi.org/10.3390/v14122794
Chicago/Turabian StylePoodts, Joaquín, Ignacio Smith, Joaquín Manuel Birenbaum, María Sol Rodriguez, Luciano Montero, Federico Javier Wolman, Juan Ignacio Marfía, Silvina Noemí Valdez, Leonardo Gabriel Alonso, Alexandra Marisa Targovnik, and et al. 2022. "Improved Expression of SARS-CoV-2 Spike RBD Using the Insect Cell-Baculovirus System" Viruses 14, no. 12: 2794. https://doi.org/10.3390/v14122794
APA StylePoodts, J., Smith, I., Birenbaum, J. M., Rodriguez, M. S., Montero, L., Wolman, F. J., Marfía, J. I., Valdez, S. N., Alonso, L. G., Targovnik, A. M., & Miranda, M. V. (2022). Improved Expression of SARS-CoV-2 Spike RBD Using the Insect Cell-Baculovirus System. Viruses, 14(12), 2794. https://doi.org/10.3390/v14122794