Anticoronavirus Evaluation of Antimicrobial Diterpenoids: Application of New Ferruginol Analogues
Abstract
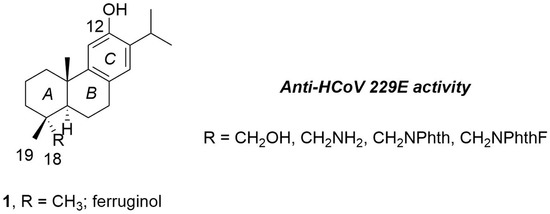
:1. Introduction
2. Materials and Methods
2.1. Chemistry: General Experimental Procedures
2.2. Synthesis
2.3. Anti-HCoV 229E Assay
2.3.1. Cell Culture and Virus
2.3.2. Cytotoxicity
2.3.3. Antiviral Assay
2.4. In Silico Simulations
2.4.1. Calculation of Molecular Properties (Drug-Likeness)
2.4.2. Toxicity Prediction (GUSAR)
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, X.; Li, Z. Medicinal chemistry strategies toward host targeting antiviral agents. Med. Res. Rev. 2020, 40, 1519–1557. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Trans. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thabti, I.; Albert, Q.; Philippot, S.; Dupire, F.; Westerhuis, B.; Fontanay, S.; Risler, A.; Kassab, T.; Elfalleh, W.; Aferchichi, A.; et al. Advances on antiviral activity of Morus spp. plant extracts: Human coronavirus and virus-related respiratory tract infections in the spotlight. Molecules 2020, 25, 1876. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 25 May 2023).
- Dong, J.; Varbanov, M.; Philippot, S.; Vreken, F.; Zeng, W.-B.; Blay, V. Ligand-based discovery of coronavirus main protease inhibitors using MACAW molecular embeddings. J. Enzym. Inhib. Med. Chem. 2023, 38, 24–35. [Google Scholar] [CrossRef]
- Wanounou, M.; Caraco, Y.; Levy, R.H.; Bialer, M.; Perucca, E. Clinically relevant interactions between ritonavir-boosted nirmatrelvir and concomitant antiseizure medications: Implications for the management of COVID-19 in patients with epilepsy. Clin. Pharmacokinet. 2022, 61, 1219–1236. [Google Scholar] [CrossRef]
- Vougogiannopoulou, K.; Corona, A.; Tramontano, E.; Alexis, N.M.; Skaltsounis, A.-L. Natural and nature-derived products targeting human coronaviruses. Molecules 2021, 26, 448. [Google Scholar] [CrossRef]
- Wardana, A.P.; Aminah, N.S.; Rosyda, M.; Abdjan, M.I.; Kristanti, A.N.; Tun, K.N.W.; Choudhary, M.I.; Takaya, Y. Potential of diterpene compounds as antivirals, a review. Heliyon 2021, 7, e07777. [Google Scholar] [CrossRef]
- Jantan, I.; Arshad, L.; Septama, A.W.; Haque, M.A.; Mohamed-Hussein, Z.-A.; Govender, N.T. Antiviral effects of phytochemicals against severe acute respiratory syndrome coronavirus and their mechanisms of action: A review. Phytother. Res. 2023, 37, 1036–1056. [Google Scholar] [CrossRef]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- González, M.A. Synthetic derivatives of aromatic abietane diterpenoids and their biological activities. Eur. J. Med. Chem. 2014, 87, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Lee, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S.; et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome Coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.-Y.; Kim, D.; Naguyen, T.T.H.; Park, S.-J.; Chang, J.S.; Park, K.H.; et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef] [PubMed]
- Roa-Linares, V.C.; Brand, Y.M.; Agudelo-Gomez, L.S.; Tangarife-Castaño, V.; Betancur-Galvis, L.A.; Gallego-Gomez, J.C.; González, M.A. Anti-herpetic and anti-dengue activity of abietane ferruginol analogues synthesized from (+)-dehydroabietylamine. Eur. J. Med. Chem. 2016, 108, 79–88. [Google Scholar] [CrossRef]
- Sousa, F.T.G.; Nunes, C.; Romano, C.M.; Sabino, E.C.; González-Cardenete, M.A. Anti-Zika virus activity of several abietane-type ferruginol analogues. Rev. Inst. Med. Trop. São Paulo 2020, 62, e97. [Google Scholar] [CrossRef] [PubMed]
- González-Cardenete, M.A.; Hamulić, D.; Miquel-Leal, F.J.; González-Zapata, N.; Jimenez-Jarava, O.J.; Brand, Y.M.; Restrepo-Mendez, L.C.; Martinez-Gutierrez, M.; Betancur-Galvis, L.A.; Marín, M.L. Antiviral profiling of C18- or C19-functionalized semisynthetic abietane diterpenoids. J. Nat. Prod. 2022, 85, 2044–2051. [Google Scholar] [CrossRef]
- Pariš, A.; Štrukelj, B.; Renko, M.; Turk, V.; Pukl, M.; Umek, A.; Korant, B.D. Inhibitory effect of carnosolic acid on HIV-1 protease in cell-free assays. J. Nat. Prod. 1993, 56, 1426–1430. [Google Scholar] [CrossRef]
- Shin, H.-B.; Choi, M.-S.; Ryu, B.; Lee, N.-R.; Kim, H.-I.; Choi, H.-E.; Chang, J.; Lee, K.-T.; Jang, D.S.; Inn, K.-S. Antiviral activity of carnosic acid against respiratory syncytial virus. Virol. J. 2013, 10, 303. [Google Scholar] [CrossRef] [Green Version]
- Gigante, B.; Santos, C.; Silva, A.; Curto, M.; Nascimento, M.; Pinto, E.; Pedro, M.; Cerqueira, F.; Pinto, M.; Duarte, M.; et al. Catechols from Abietic Acid: Synthesis and Evaluation as Bioactive Compounds. Bioorg. Med. Chem. 2003, 11, 1631–1638. [Google Scholar] [CrossRef]
- González, M.A.; Pérez-Guaita, D.; Correa-Royero, J.; Zapata, B.; Agudelo, L.; Mesa-Arango, A.; Betancur-Galvis, L. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur. J. Med. Chem. 2010, 45, 811–816. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Li, Y.-H.; Jiang, J.-D.; Yu, S.-S.; Qu, J.; Ma, S.-G.; Liu, Y.-B.; Yu, D.-Q. Anti-Coxsackie virus B diterpenes from the roots of Illicium jiadifengpi. Tetrahedron 2013, 69, 1017–1023. [Google Scholar] [CrossRef]
- González, M.A.; Pérez-Guaita, D. Short syntheses of (+)-ferruginol from (+)-dehydroabietylamine. Tetrahedron 2012, 68, 9612–9615. [Google Scholar] [CrossRef]
- Hamulić, D.; Stadler, M.; Hering, S.; Padrón, J.M.; Bassett, R.; Rivas, F.; Loza-Mejía, M.A.; Dea-Ayuela, M.A.; González-Cardenete, M.A. Synthesis and biological studies of (+)-Liquiditerpenoic acid A (abietopinoic Acid) and representative analogues: SAR studies. J. Nat. Prod. 2019, 82, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malkowsky, I.M.; Nieger, M.; Kataeva, O.; Waldvogel, S.R. Synthesis and properties of optically pure phenols derived from (+)-dehydroabietylamine. Synthesis 2007, 2007, 773–778. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lagunin, A.; Zakharov, A.; Filimonov, D.; Poroikov, V. QSAR modeling of rat acute toxicity on the basis of PASS prediction. Mol. Inform. 2011, 30, 241–250. [Google Scholar] [CrossRef]
- Canadian Center for Occupational Health and Safety. What Is an LD50 and LC50? Available online: http://www.ccohs.ca/oshanswers/chemicals/LD50.html#_1_6 (accessed on 28 January 2023).
- Sadym, A.; Lagunin, A.; Filimonov, D.; Poroikov, V. Prediction of biological activity spectra via the Internet. SAR QSAR Environ. Res. 2003, 14, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Poroikov, V.V.; Filimonov, D.A.; Ihlenfeld, W.-D.; Gloriozova, T.A.; Lagunin, A.A.; Borodina, Y.B.; Stepanchikova, A.V.; Nicklaus, M.C. PASS biological activity spectrum predictions in the enhanced open NCI database browser. J. Chem. Inf. Comput. Sci. 2003, 43, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Denaro, M.; Smeriglio, A.; Barreca, D.; De Francesco, C.; Occhiuto, C.; Milano, G.; Trombetta, D. Antiviral activity of plants and their isolated bioactive compounds: An update. Phytother. Res. 2020, 34, 742–768. [Google Scholar] [CrossRef]
- Islam, M.T.; Sarkar, C.; El-Kersh, D.M.; Jamaddar, S.; Uddin, S.J.; Shilpi, J.A.; Mubarak, M.S. Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phytother. Res. 2020, 34, 2471–2492. [Google Scholar] [CrossRef]
- Verma, S.; Twilley, D.; Esmear, T.; Oosthuizen, C.B.; Reid, A.-M.; Nel, M.; Lall, N. Anti-SARS-CoV natural products with the potential to inhibit SARS-CoV-2 (COVID-19). Front. Pharmacol. 2020, 11, 561334. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil –new insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Perez-Castillo, Y.; Elshabrawy, H.A.; Bezerra-Filho, C.S.M.; Pergentino de Sousa, D. Bioactive terpenes and their derivatives as potential SARS-CoV-2 proteases inhibitors from molecular modeling studies. Biomolecules 2021, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.-T.; Cheng, T.-J.R.; Juang, Y.-P.; Ma, H.-H.; Wu, Y.-T.; Yang, W.-B.; Cheng, C.-W.; Chen, X.; Chou, T.-H.; Shie, J.-J.; et al. Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e20211579118. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, J.H.; Kim, Y.M.; Jeong, H.J.; Kim, D.W.; Park, K.H.; Kwon, H.-J.; Park, S.-J.; Lee, W.S.; Ryu, Y.B. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012, 20, 5928–5935. [Google Scholar] [CrossRef]
- Elebeedy, D.; Elkhatib, W.F.; Kandeil, A.; Ghanem, A.; Kutkat, O.; Alnajjar, R.; Saleh, M.A.; El Maksoud, A.I.A.; Badawy, I.; Al-Karmalawy, A.A. Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational and in vitro insidghts. RSC Adv. 2021, 11, 29267–29286. [Google Scholar] [CrossRef]
- Tret’yakova, E.V.; Ma, X.; Kazakova, O.B.; Shtro, A.A.; Petukhova, G.D.; Klabukov, A.M.; Dyatlov, D.S.; Smirnova, A.A.; Xu, H.; Xiao, S. Synthesis and evaluation of diterpenic mannich bases as antiviral agents against influenza A and SARS-CoV-2. Phytochem. Lett. 2022, 51, 91–96. [Google Scholar] [CrossRef] [PubMed]





| Compound | miLog P | MW | n-HBA | n-HBD | TPSA | Lipinski’s Violation |
|---|---|---|---|---|---|---|
| 1 | 6.41 | 286.46 | 1 | 1 | 20.23 | 1 |
| 3 | 7.08 | 431.58 | 4 | 1 | 59.30 | 1 |
| 12 | 5.24 | 302.46 | 2 | 2 | 40.46 | 1 |
| 13 | 4.33 | 316.44 | 3 | 2 | 57.53 | 0 |
| 14 | 4.67 | 301.47 | 2 | 3 | 46.25 | 0 |
| 15 | 6.88 | 449.57 | 4 | 1 | 59.30 | 1 |
| 16 | 6.25 | 449.57 | 4 | 1 | 59.30 | 1 |
| Rule of five | not >5 | <500 | not >10 | not >5 | 1 violation allowed |
| Acute Rat Toxicity Parameters | Compounds | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 12 | 13 | 14 | 15 | 16 | |
| Rat LD50 Values | |||||||
| Rat IP a LD50 (mg/kg) | 0.00 | 1820.00 | 847.50 | 807.50 | 607.20 | 567.60 | 657.60 |
| Rat IV b LD50 (mg/kg) | 0.00 | 30.90 | 33.52 | 34.50 | 17.84 | 24.57 | 32.71 |
| Rat Oral c LD50 (mg/kg) | 0.00 | 1015.00 | 2175.00 | 2639.00 | 2167.00 | 1385.00 | 1429.00 |
| Rat SC d LD50 (mg/kg) | 0.00 | 400.80 | 602.90 | 295.00 | 882.00 | 227.30 | 420.30 |
| Acute rodent toxicity classification of compounds by OECD project | |||||||
| Rat IP LD50 Classification | Class 1 | Non-toxic | Class 5 | Class 5 | Class 5 | Class 5 | Class 5 |
| Rat IV LD50 Classification | Class 1 | Class 1 | Class 3 | Class 3 | Class 3 | Class 3 | Class 3 |
| Rat Oral LD50 Classification | Class 1 | Class 4 | Class 5 | Class 5 | Class 5 | Class 4 | Class 4 |
| Rat SC LD50 Classification | Class 1 | Class 4 | Class 4 | Class 4 | Class 4 | Class 4 | Class 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varbanov, M.; Philippot, S.; González-Cardenete, M.A. Anticoronavirus Evaluation of Antimicrobial Diterpenoids: Application of New Ferruginol Analogues. Viruses 2023, 15, 1342. https://doi.org/10.3390/v15061342
Varbanov M, Philippot S, González-Cardenete MA. Anticoronavirus Evaluation of Antimicrobial Diterpenoids: Application of New Ferruginol Analogues. Viruses. 2023; 15(6):1342. https://doi.org/10.3390/v15061342
Chicago/Turabian StyleVarbanov, Mihayl, Stéphanie Philippot, and Miguel A. González-Cardenete. 2023. "Anticoronavirus Evaluation of Antimicrobial Diterpenoids: Application of New Ferruginol Analogues" Viruses 15, no. 6: 1342. https://doi.org/10.3390/v15061342
APA StyleVarbanov, M., Philippot, S., & González-Cardenete, M. A. (2023). Anticoronavirus Evaluation of Antimicrobial Diterpenoids: Application of New Ferruginol Analogues. Viruses, 15(6), 1342. https://doi.org/10.3390/v15061342