Digital PCR (dPCR) Quantification of miR-155-5p as a Potential Candidate for a Tissue Biomarker of Inflammation in Rabbits Infected with Lagovirus europaeus/Rabbit Hemorrhagic Disease Virus (RHDV)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Animals
2.1.2. Lagovirus europaeus/RHDV
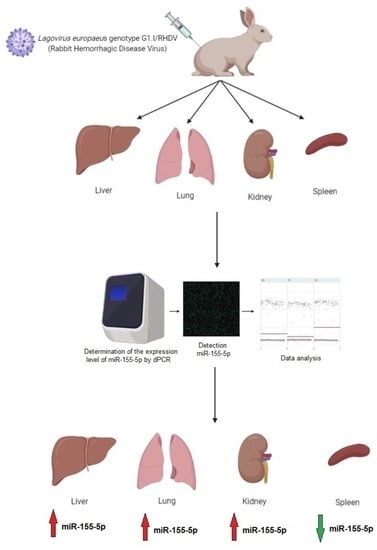
2.1.3. Tissue Samples
2.2. Methods
2.2.1. MiRNA Isolation from the Tissue Samples and Reverse Transcription Reaction
2.2.2. Quantification of Ocu-miR-155-5p in Tissue Samples Using dPCR
2.2.3. Statistics
3. Results
3.1. Absolute Quantification of Ocu-miRNA-155-5p by dPCR in Rabbits Infected with L. europaeus/RHDV
3.2. Clinical Signs of Lagovirus europaeus/RHDV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar] [PubMed]
- Ostrycharz, E.; Hukowska-Szematowicz, B. Micro-Players of Great Significance-Host microRNA Signature in Viral Infections in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 536. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Naseri, A.; Shojaie, L.; Nemati, M.; Jafarzadeh, S.; Bannazadeh Baghi, H.; Hamblin, M.R.; Akhlagh, S.A.; Mirzaei, H. MicroRNA-155 and antiviral immune responses. Int. Immunopharmacol. 2021, 101, 108188. [Google Scholar] [CrossRef]
- Zeng, F.R.; Tang, L.J.; He, Y.; Garcia, R.C. An update on the role of miRNA-155 in pathogenic microbial infections. Microbes Infect. 2015, 17, 613–621. [Google Scholar] [CrossRef]
- Marques-Rocha, J.L.; Samblas, M.; Milagro, F.I.; Bressan, J.; Martinez, J.A.; Marti, A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015, 29, 3595–3611. [Google Scholar] [CrossRef] [Green Version]
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef]
- Vigorito, E.; Kohlhaas, S.; Lu, D.; Leyland, R. miR-155: An ancient regulator of the immune system. Immunol. Rev. 2013, 253, 146–157. [Google Scholar] [CrossRef]
- Goncalves-Alves, E.; Saferding, V.; Schliehe, C.; Benson, R.; Kurowska-Stolarska, M.; Brunner, J.S.; Puchner, A.; Podesser, B.K.; Smolen, J.S.; Redlich, K.; et al. MicroRNA-155 Controls T Helper Cell Activation During Viral Infection. Front. Immunol. 2019, 10, 1367. [Google Scholar] [CrossRef] [Green Version]
- Le Pendu, J.; Abrantes, J.; Bertagnoli, S.; Guitton, J.S.; Le Gall-Recule, G.; Lopes, A.M.; Marchandeau, S.; Alda, F.; Almeida, T.; Celio, A.P.; et al. Proposal for a unified classification system and nomenclature of lagoviruses. J. Gen. Virol. 2017, 98, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, J.; van der Loo, W.; Le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.J.; Xue, H.P.; Pu, B.Q.; Qian, N.H. A new viral disease in rabbits. Anim. Husb. Vet. Med. 1984, 16, 253–255. [Google Scholar]
- Marcato, P.S.; Benazzi, C.; Vecchi, G.; Galeotti, M.; Della Salda, L.; Sarli, G.; Lucidi, P. Clinical and pathological features of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev. Sci. Tech. 1991, 10, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, J.; Lopes, A.M. A Review on the Methods Used for the Detection and Diagnosis of Rabbit Hemorrhagic Disease Virus (RHDV). Microorganisms 2021, 9, 972. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.G.; Costa, E.S.A.; Oliveira, M.J.; Monteiro, E.; Aguas, A.P. Leukocyte-hepatocyte interaction in calicivirus infection: Differences between rabbits that are resistant or susceptible to rabbit haemorrhagic disease (RHD). Vet. Immunol. Immunopathol. 2005, 103, 217–221. [Google Scholar] [CrossRef]
- Mitro, S.; Krauss, H. Rabbit hemorrhagic disease: A review with special reference to its epizootiology. Eur. J. Epidemiol. 1993, 9, 70–78. [Google Scholar] [CrossRef]
- Xu, Z.J.; Chen, W.X. Viral haemorrhagic disease in rabbits: A review. Vet. Res. Commun. 1989, 13, 205–212. [Google Scholar] [CrossRef]
- Alonso, C.; Oviedo, J.M.; Martin-Alonso, J.M.; Diaz, E.; Boga, J.A.; Parra, F. Programmed cell death in the pathogenesis of rabbit hemorrhagic disease. Arch. Virol. 1998, 143, 321–332. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, B.J.; Tai, J.H.; Park, J.H.; Lee, Y.S. Apoptosis in rabbit haemorrhagic disease. J. Comp. Pathol. 2000, 123, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, Y.S.; Itakura, C. Pathogenesis of acute necrotic hepatitis in rabbit hemorrhagic disease. Lab. Anim. Sci. 1995, 45, 445–449. [Google Scholar] [PubMed]
- Niedźwiedzka-Rystwej, P.; Deptuła, W. Lymphocyte subpopulations and apoptosis of immune cells in rabbits experimentally infected with a strain of the RHD virus having a variable haemagglutination capacity. Pol. J. Vet. Sci. 2012, 15, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedzka-Rystwej, P.; Deptuła, W. Apoptosis of peripheral blood leukocytes from rabbits infected with non-haemagglutinating strains of rabbit haemorrhagic disease virus (RHDV). Vet. Immunol. Immunopathol. 2012, 149, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Hukowska-Szematowicz, B. Genetic and immunological characteristic of European strains of RHD (rabbit haemorrhagic disease) virus. Pol. J. Environ. Stud. 2013, 2, 1–114. [Google Scholar]
- Niedźwiedzka-Rystwej, P.; Hukowska-Szematowicz, B.; Tokarz-Deptuła, B.; Trzeciak-Ryczek, A.; Działo, J.; Deptuła, W. Apoptosis of peripheral blood leucocytes in rabbits infected with different strains of rabbit haemorrhagic disease virus. Acta Biochim. Pol. 2013, 60, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Marques, R.M.; Costa, E.S.A.; Aguas, A.P.; Teixeira, L.; Ferreira, P.G. Early acute depletion of lymphocytes in calicivirus-infected adult rabbits. Vet. Res. Commun. 2010, 34, 659–668. [Google Scholar] [CrossRef]
- Teixeira, L.; Marques, R.M.; Aguas, A.P.; Ferreira, P.G. Regulatory T cells are decreased in acute RHDV lethal infection of adult rabbits. Vet. Immunol. Immunopathol. 2012, 148, 343–347. [Google Scholar] [CrossRef]
- Tokarz-Deptuła, B. Immunity phenomena in rabbits infected with the RHD virus (rabbit haemorrhagic disease). Pol. J. Environ. Sci. 2009, 7, 1–81. [Google Scholar]
- Niedźwiedzka-Rystwej, P.; Tokarz-Deptuła, B.; Hukowska-Szematowicz, B.; Działo, J.; Deptuła, W. Indices of non-specific immunity: An element of natural immunity in rabbits infected with RHD (rabbit haemorrhagic disease) virus. Cent. Eur. J. Immunol. 2013, 38, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Hukowska-Szematowicz, B.; Deptuła, W. Non-specific cellular in rabbits experimentlaly infected with four Czech strains of the Rabbit Haemorrhagic Disease Virus with Different patho-genicity. Pol. J. Environ. Stud. 2012, 21, 879–892. [Google Scholar]
- Hukowska-Szematowicz, B.; Deptuła, W. Peripheral blood lymphocytes in rabbits infected with Czech strains, CAMPV-562 and CAMPV-558 of RHD virus. Cent. Eur. J. Immunol. 2008, 33, 8–13. [Google Scholar]
- Niedźwiedzka-Rystwej, P.; Deptuła, W. Non-specyfic immunity in rabbits infected with 10 strain of the rabbit haemorrhagic disease virus with different biological properties. Cent. Eur. J. Biol. 2012, 5, 613–632. [Google Scholar] [CrossRef]
- Trzeciak-Ryczek, A.; Tokarz-Deptula, B.; Deptula, W. Expression of IL-1beta, IL-2, IL-10, TNF-beta and GM-CSF in peripheral blood leukocytes of rabbits experimentally infected with rabbit haemorrhagic disease virus. Vet. Microbiol. 2016, 186, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak-Ryczek, A.; Tokarz-Deptuła, B.; Deptuła, W. Expression of IL-1Ra, IL-6, IL-8, IL-18, TNF-alpha and IFN-gamma genes in peripheral blood leukocytes of rabbits infected with RHDV (Rabbit Haemorrhagic Disease Virus). Dev. Comp. Immunol. 2017, 76, 310–315. [Google Scholar] [CrossRef]
- Semerjyan, A.B.; Sargsyan, M.A.; Arzumanyan, H.H.; Hakobyan, L.H.; Abroyan, L.O.; Semerjyan, Z.B.; Avetisyan, A.S.; Karalova, E.M.; Manukyan, D.M.; Matevosyan, H.S.; et al. Immune cell pathology in rabbit hemorrhagic disease. Vet. World 2019, 12, 1332–1340. [Google Scholar] [CrossRef] [Green Version]
- Marques, R.M.; Costa, E.S.A.; Aguas, A.P.; Teixeira, L.; Ferreira, P.G. Early inflammatory response of young rabbits attending natural resistance to calicivirus (RHDV) infection. Vet. Immunol. Immunopathol. 2012, 150, 181–188. [Google Scholar] [CrossRef]
- O’Toole, A.D.; Mohamed, F.M.; Zhang, J.; Brown, C.C. Early pathogenesis in rabbit hemorrhagic disease virus 2. Microb. Pathog. 2022, 173, 105814. [Google Scholar] [CrossRef]
- Ostrycharz, E.; Blatkiewicz, M.; Hukowska-Szematowicz, B. Młodzi Naukowcy 2.0. Tom 1. Expression of the Master Anti-Inflammatory Regulator IL-10 during Lagovirus Europaeus GI.1/RHDV (Rabbit Hemorrhagic Disease Virus) Infection; Korpysa, J., Niedźwiedzka-Rystwej, P., Eds.; Fundacji Centrum Badań Socjologicznych (Centre of Sociological Research): Szczecin, Poland, 2021; pp. 59–72. Available online: https://www.csr-pub.eu/?41,en_mlodzi-naukowcy-2.0 (accessed on 7 July 2023).
- Hukowska-Szematowicz, B.; Maciejak-Jastrzębska, A.; Blatkiewicz, M.; Maciak, K.; Góra, M.; Janiszewska, J.; Burzyńska, B. Changes in MicroRNA Expression during Rabbit Hemorrhagic Disease Virus (RHDV) Infection. Viruses 2020, 12, 965. [Google Scholar] [CrossRef]
- Lawrie, C.H.; Gal, S.; Dunlop, H.M.; Pushkaran, B.; Liggins, A.P.; Pulford, K.; Banham, A.H.; Pezzella, F.; Boultwood, J.; Wainscoat, J.S.; et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br. J. Haematol. 2008, 141, 672–675. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Sheneef, A.; Gouda, A.M.; Mohammad, A.N.; Yousef, L.M.; Noureldin, A.K. Serum MicroRNA-122 and MicroRNA-155: Markers of Disease Progression in Hepatitis C viral infection. Egypt. J. Immunol. 2017, 24, 33–46. [Google Scholar] [PubMed]
- Donyavi, T.; Bokharaei-Salim, F.; Baghi, H.B.; Khanaliha, K.; Alaei Janat-Makan, M.; Karimi, B.; Sadri Nahand, J.; Mirzaei, H.; Khatami, A.; Garshasbi, S.; et al. Acute and post-acute phase of COVID-19: Analyzing expression patterns of miRNA-29a-3p, 146a-3p, 155–5p, and let-7b-3p in PBMC. Int. Immunopharmacol. 2021, 97, 107641. [Google Scholar] [CrossRef] [PubMed]
- Giannella, A.; Riccetti, S.; Sinigaglia, A.; Piubelli, C.; Razzaboni, E.; Di Battista, P.; Agostini, M.; Dal Molin, E.; Manganelli, R.; Gobbi, F.; et al. Circulating microRNA signatures associated with disease severity and outcome in COVID-19 patients. Front. Immunol. 2022, 13, 968991. [Google Scholar] [CrossRef] [PubMed]
- Baluni, M.; Ghildiyal, S.; Fatima, T.; Tiwari, R.; Upadhyay, S.; Dhole, T.N.; Reddy, D.H.; Singh, D. Differential expression of circulating microRNAs in serum: Potential biomarkers to track Japanese encephalitis virus infection. J. Med. Virol. 2022, 94, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Moghoofei, M.; Najafipour, S.; Mostafaei, S.; Tavakoli, A.; Bokharaei-Salim, F.; Ghorbani, S.; Javanmard, D.; Ghaffari, H.; Monavari, S.H. MicroRNAs Profiling in HIV, HCV, and HIV/HCV Co-Infected Patients. Curr. HIV Res. 2021, 19, 27–34. [Google Scholar] [CrossRef]
- Fang, J.; Zhuge, L.; Rao, H.; Huang, S.; Jin, L.; Li, J. Increased Levels of miR-155 are Related to Higher T-Cell Activation in the Peripheral Blood of Patients with Chronic Hepatitis B. Genet. Test. Mol. Biomarkers 2019, 23, 118–123. [Google Scholar] [CrossRef]
- Abbasi-Kolli, M.; Sadri Nahand, J.; Kiani, S.J.; Khanaliha, K.; Khatami, A.; Taghizadieh, M.; Torkamani, A.R.; Babakhaniyan, K.; Bokharaei-Salim, F. The expression patterns of MALAT-1, NEAT-1, THRIL, and miR-155-5p in the acute to the post-acute phase of COVID-19 disease. Braz. J. Infect. Dis. 2022, 26, 102354. [Google Scholar] [CrossRef]
- Kaluzna, E.M. MicroRNA-155 and microRNA-196b: Promising biomarkers in hepatitis C virus infection? Rev. Med. Virol. 2014, 24, 169–185. [Google Scholar] [CrossRef]
- Kassif-Lerner, R.; Zloto, K.; Rubin, N.; Asraf, K.; Doolman, R.; Paret, G.; Nevo-Caspi, Y. miR-155: A Potential Biomarker for Predicting Mortality in COVID-19 Patients. J. Pers. Med. 2022, 12, 324. [Google Scholar] [CrossRef]
- Haroun, R.A.; Osman, W.H.; Amin, R.E.; Hassan, A.K.; Abo-Shanab, W.S.; Eessa, A.M. Circulating plasma miR-155 is a potential biomarker for the detection of SARS-CoV-2 infection. Pathology 2022, 54, 104–110. [Google Scholar] [CrossRef]
- Cui, C.; Cui, Q. The relationship of human tissue microRNAs with those from body fluids. Sci. Rep. 2020, 10, 5644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Ma, J.; Guarnera, M.A.; Fang, H.; Cai, L.; Jiang, F. Digital PCR quantification of miRNAs in sputum for diagnosis of lung cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Li, N.; Guarnera, M.; Jiang, F. Quantification of Plasma miRNAs by Digital PCR for Cancer Diagnosis. Biomark. Insights 2013, 8, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.C.; Lai, Y.T.; Rahman, M.M.; Chen, H.W.; Husna, A.A.; Fujikawa, T.; Ando, T.; Kitahara, G.; Koiwa, M.; Kubota, C.; et al. Bovine milk transcriptome analysis reveals microRNAs and RNU2 involved in mastitis. FEBS J. 2020, 287, 1899–1918. [Google Scholar] [CrossRef]
- Raoof, R.; Jimenez-Mateos, E.M.; Bauer, S.; Tackenberg, B.; Rosenow, F.; Lang, J.; Onugoren, M.D.; Hamer, H.; Huchtemann, T.; Kortvelyessy, P.; et al. Cerebrospinal fluid microRNAs are potential biomarkers of temporal lobe epilepsy and status epilepticus. Sci. Rep. 2017, 7, 3328. [Google Scholar] [CrossRef] [Green Version]
- Benning, L.; Robinson, S.; Follo, M.; Heger, L.A.; Stallmann, D.; Duerschmied, D.; Bode, C.; Ahrens, I.; Hortmann, M. Digital PCR for Quantifying Circulating MicroRNAs in Acute Myocardial Infarction and Cardiovascular Disease. J. Vis. Exp. 2018, 137, e57950. [Google Scholar] [CrossRef]
- Llano-Diez, M.; Ortez, C.I.; Gay, J.A.; Alvarez-Cabado, L.; Jou, C.; Medina, J.; Nascimento, A.; Jimenez-Mallebrera, C. Digital PCR quantification of miR-30c and miR-181a as serum biomarkers for Duchenne muscular dystrophy. Neuromuscul. Disord. 2017, 27, 15–23. [Google Scholar] [CrossRef]
- Songia, P.; Chiesa, M.; Valerio, V.; Moschetta, D.; Myasoedova, V.A.; D’Alessandra, Y.; Poggio, P. Direct screening of plasma circulating microRNAs. RNA Biol. 2018, 15, 1268–1272. [Google Scholar] [CrossRef] [Green Version]
- Kopkova, A.; Sana, J.; Fadrus, P.; Machackova, T.; Vecera, M.; Vybihal, V.; Juracek, J.; Vychytilova-Faltejskova, P.; Smrcka, M.; Slaby, O. MicroRNA isolation and quantification in cerebrospinal fluid: A comparative methodical study. PLoS ONE 2018, 13, e0208580. [Google Scholar] [CrossRef] [Green Version]
- Baker, M. Digital PCR hits its stride. Nat. Methods 2012, 9, 541–544. [Google Scholar] [CrossRef]
- Day, E.; Dear, P.H.; McCaughan, F. Digital PCR strategies in the development and analysis of molecular biomarkers for personalized medicine. Methods 2013, 59, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Morley, A.A. Digital PCR: A brief history. Biomol. Detect. Quantif. 2014, 1, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, P.L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Cui, X.; Hu, J.; Li, Z.; Choi, J.R.; Yang, Q.; Lin, M.; Ying Hui, L.; Xu, F. Advances in digital polymerase chain reaction (dPCR) and its emerging biomedical applications. Biosens. Bioelectron. 2017, 90, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 2013, 10, 1003–1005. [Google Scholar] [CrossRef]
- Blatkiewicz, M.; Hukowska-Szematowicz, B. Vimentin as a Cap of Invisibility: Proposed Role of Vimentin in Rabbit Hemorrhagic Disease Virus (RHDV) Infection. Viruses 2021, 13, 1416. [Google Scholar] [CrossRef]
- Materiały Informacyjno-Szkoleniowe Sekcji ds. Zwierząt Laboratoryjnych; Information and training materials of the Laboratory Animals Section, General Assembly of the Association of Agriculture Engineers and Technicians; ZG Stowarzyszenia Inżynierów i Techników Rolnictwa: Warsaw, Poland, 1987; pp. 26–77. (In Polish) [Google Scholar]
- Regulation of the Minister of Agriculture and Rural Development of 10 March 2006 on Detailed Conditions for Maintenance of Laboratory Animals in Experimental Units, Breeding Units and Suppliers (Polish Journal of Laws of 2006, No 50, Item 368). Available online: https://leap.unep.org/countries/pl/national-legislation/regulation-detailed-conditions-keeping-laboratory-animals (accessed on 12 July 2023).
- Fitzner, A.; Kęsy, A.; Niedbalski, W.; Paprocka, G.; Walkowiak, B. Identification of the dominating VP60 polypeptide in domestic isolates of the RHD virus. Med. Wet. 1996, 52, 303–305. [Google Scholar]
- Faraoni, I.; Antonetti, F.R.; Cardone, J.; Bonmassar, E. miR-155 gene: A typical multifunctional microRNA. Biochim. Biophys. Acta 2009, 1792, 497–505. [Google Scholar] [CrossRef]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef]
- Huggett, J.F.; Whale, A. Digital PCR as a novel technology and its potential implications for molecular diagnostics. Clin. Chem. 2013, 59, 1691–1693. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, R.H.; Jerome, K.R. Viral diagnostics in the era of digital polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 2013, 75, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Fan, D.; Wang, N.; Wang, F.; Wang, B.; Zhu, L.; Guo, Y. Applications of digital PCR in COVID-19 pandemic. View 2021, 2, 20200082. [Google Scholar] [CrossRef] [PubMed]
- Hijano, D.R.; Brazelton de Cardenas, J.; Maron, G.; Garner, C.D.; Ferrolino, J.A.; Dallas, R.H.; Gu, Z.; Hayden, R.T. Clinical correlation of influenza and respiratory syncytial virus load measured by digital PCR. PLoS ONE 2019, 14, e0220908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyaruaba, R.; Mwaliko, C.; Dobnik, D.; Neuzil, P.; Amoth, P.; Mwau, M.; Yu, J.; Yang, H.; Wei, H. Digital PCR Applications in the SARS-CoV-2/COVID-19 Era: A Roadmap for Future Outbreaks. Clin. Microbiol. Rev. 2022, 35, e0016821. [Google Scholar] [CrossRef]
- Rutsaert, S.; Bosman, K.; Trypsteen, W.; Nijhuis, M.; Vandekerckhove, L. Digital PCR as a tool to measure HIV persistence. Retrovirology 2018, 15, 16. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Ding, C.; Chen, Q.; Xie, J.; Yu, J.; Shi, Y.; Jiang, C.; Zhang, Z.; He, H.; Ge, Y.; et al. Digital PCR assay for the effective detection of COVID-19 patients with SARS-CoV-2 low viral load. J. Virol. Methods 2021, 295, 114185. [Google Scholar] [CrossRef]
- Poggio, P.; Songia, P.; Vavassori, C.; Ricci, V.; Banfi, C.; Barbieri, S.S.; Garoffolo, G.; Myasoedova, V.A.; Piacentini, L.; Raucci, A.; et al. Digital PCR for high sensitivity viral detection in false-negative SARS-CoV-2 patients. Sci. Rep. 2021, 11, 4310. [Google Scholar] [CrossRef]
- Polo, D.; Schaeffer, J.; Fournet, N.; Le Saux, J.C.; Parnaudeau, S.; McLeod, C.; Le Guyader, F.S. Digital PCR for Quantifying Norovirus in Oysters Implicated in Outbreaks, France. Emerg. Infect. Dis. 2016, 22, 2189–2191. [Google Scholar] [CrossRef]
- Dong, L.; Zhou, J.; Niu, C.; Wang, Q.; Pan, Y.; Sheng, S.; Wang, X.; Zhang, Y.; Yang, J.; Liu, M.; et al. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta 2021, 224, 121726. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Blaya, D.; Aguilar-Bravo, B.; Hao, F.; Casacuberta-Serra, S.; Coll, M.; Perea, L.; Vallverdu, J.; Graupera, I.; Pose, E.; Llovet, L.; et al. Expression of microRNA-155 in inflammatory cells modulates liver injury. Hepatology 2018, 68, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef] [PubMed]
- Neimanis, A.; Larsson Pettersson, U.; Huang, N.; Gavier-Widen, D.; Strive, T. Elucidation of the pathology and tissue distribution of Lagovirus europaeus GI.2/RHDV2 (rabbit haemorrhagic disease virus 2) in young and adult rabbits (Oryctolagus cuniculus). Vet. Res. 2018, 49, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalton, K.P.; Balseiro, A.; Juste, R.A.; Podadera, A.; Nicieza, I.; Del Llano, D.; Gonzalez, R.; Martin Alonso, J.M.; Prieto, J.M.; Parra, F.; et al. Clinical course and pathogenicity of variant rabbit haemorrhagic disease virus in experimentally infected adult and kit rabbits: Significance towards control and spread. Vet. Microbiol. 2018, 220, 24–32. [Google Scholar] [CrossRef]
- Nejad, C.; Stunden, H.J.; Gantier, M.P. A guide to miRNAs in inflammation and innate immune responses. FEBS J. 2018, 285, 3695–3716. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Ge, W.; Ma, Y.; Xie, G.; Wang, W.; Han, L.; Bian, B.; Li, L.; Shen, L. miR-155 Regulates IL-10-Producing CD24(hi)CD27(+) B Cells and Impairs Their Function in Patients with Crohn’s Disease. Front. Immunol. 2017, 8, 914. [Google Scholar] [CrossRef] [Green Version]
- Gaytan-Pacheco, N.; Ibanez-Salazar, A.; Herrera-Van Oostdam, A.S.; Oropeza-Valdez, J.J.; Magana-Aquino, M.; Adrian Lopez, J.; Monarrez-Espino, J.; Lopez-Hernandez, Y. miR-146a, miR-221, and miR-155 are Involved in Inflammatory Immune Response in Severe COVID-19 Patients. Diagnostics 2022, 13, 133. [Google Scholar] [CrossRef]
- Wang, H.; Peng, W.; Shen, X.; Huang, Y.; Ouyang, X.; Dai, Y. Circulating levels of inflammation-associated miR-155 and endothelial-enriched miR-126 in patients with end-stage renal disease. Braz. J. Med. Biol. Res. 2012, 45, 1308–1314. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Rudiger, T.; Hartmann, M.; Muller-Hermelink, H.K.; Marx, A. Inflammatory reactions of the spleen. Pathologe 2008, 29, 121–128. [Google Scholar] [CrossRef]
- Fujiyama, S.; Nakahashi-Oda, C.; Abe, F.; Wang, Y.; Sato, K.; Shibuya, A. Identification and isolation of splenic tissue-resident macrophage sub-populations by flow cytometry. Int. Immunol. 2019, 31, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashima, R. Physiological roles of miR-155. Immunology 2015, 145, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Burnside, J.; Morgan, R. Emerging roles of chicken and viral microRNAs in avian disease. BMC Proc. 2011, 5 (Suppl. S4), S2. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Y.; Smith, L.P.; Lawrie, C.H.; Saunders, N.J.; Watson, M.; Nair, V. Differential expression of microRNAs in Marek’s disease virus-transformed T-lymphoma cell lines. J. Gen. Virol. 2009, 90, 1551–1559. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hukowska-Szematowicz, B.; Ostrycharz, E.; Dudzińska, W.; Roszkowska, P.; Siennicka, A.; Wojciechowska-Koszko, I. Digital PCR (dPCR) Quantification of miR-155-5p as a Potential Candidate for a Tissue Biomarker of Inflammation in Rabbits Infected with Lagovirus europaeus/Rabbit Hemorrhagic Disease Virus (RHDV). Viruses 2023, 15, 1578. https://doi.org/10.3390/v15071578
Hukowska-Szematowicz B, Ostrycharz E, Dudzińska W, Roszkowska P, Siennicka A, Wojciechowska-Koszko I. Digital PCR (dPCR) Quantification of miR-155-5p as a Potential Candidate for a Tissue Biomarker of Inflammation in Rabbits Infected with Lagovirus europaeus/Rabbit Hemorrhagic Disease Virus (RHDV). Viruses. 2023; 15(7):1578. https://doi.org/10.3390/v15071578
Chicago/Turabian StyleHukowska-Szematowicz, Beata, Ewa Ostrycharz, Wioleta Dudzińska, Paulina Roszkowska, Aldona Siennicka, and Iwona Wojciechowska-Koszko. 2023. "Digital PCR (dPCR) Quantification of miR-155-5p as a Potential Candidate for a Tissue Biomarker of Inflammation in Rabbits Infected with Lagovirus europaeus/Rabbit Hemorrhagic Disease Virus (RHDV)" Viruses 15, no. 7: 1578. https://doi.org/10.3390/v15071578
APA StyleHukowska-Szematowicz, B., Ostrycharz, E., Dudzińska, W., Roszkowska, P., Siennicka, A., & Wojciechowska-Koszko, I. (2023). Digital PCR (dPCR) Quantification of miR-155-5p as a Potential Candidate for a Tissue Biomarker of Inflammation in Rabbits Infected with Lagovirus europaeus/Rabbit Hemorrhagic Disease Virus (RHDV). Viruses, 15(7), 1578. https://doi.org/10.3390/v15071578