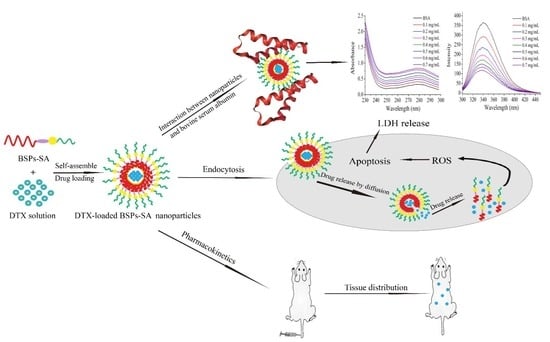
Interactions of Self-Assembled Bletilla Striata Polysaccharide Nanoparticles with Bovine Serum Albumin and Biodistribution of Its Docetaxel-Loaded Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DTX-Loaded BSPs-SA Nanoparticles
2.3. Characterization of Nanoparticles
2.3.1. Particle Size and Zeta Potential
2.3.2. Morphology and Critical Aggregation Concentration
2.3.3. Determination of Encapsulation Efficiency and Drug-Loading Capacity
2.4. Determination of Drug Release in Vitro
2.5. X-Ray Diffraction and Differential Scanning Calorimetric Analysis
2.6. Cell Viability
2.7. Assay of Lactate Dehydrogenase
2.8. Reactive Oxygen Species
2.9. Histological Examination
2.10. Fluorescence and Ultraviolet (UV) Measurement
2.11. Pharmacokinetics and Tissue Distribution
2.12. Serum and Tissue Sample Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nanoparticles
3.2. Drug Release in Vitro
3.3. X-Ray Diffraction Analysis
3.4. Differential Scanning Calorimetric Analysis
3.5. Cell Viability
3.6. Assay of Lactate Dehydrogenase
3.7. Measurement of Reactive Oxygen Species
3.8. Histological Examination
3.9. Fluorescence Spectroscopic Measurement
3.10. UV Spectroscopic Measurement
3.11. Pharmacokinetics and Tissue Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef] [PubMed]
- Cammas, S.; Suzuki, K.; Sone, C.; Sakurai, Y.; Kataoka, K.; Okano, T. Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. J. Control. Release 1997, 48, 157–164. [Google Scholar] [CrossRef]
- Feng, L.; Liu, L.; Lv, F.; Bazan, G.C.; Wang, S. Preparation and biofunctionalization of multicolor conjugated polymer nanoparticles for imaging and detection of tumor cells. Adv. Mater. 2014, 26, 3926–3930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K. Structural properties of self-assembled polymeric aggregates in aqueous solutions. Polym. Adv. Technol. 2001, 12, 2–22. [Google Scholar] [CrossRef]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007, 32, 962–990. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Hsiue, G. Polymeric micelles with a pH-responsive structure as intracellular drug carriers. J. Control. Release 2005, 108, 140–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharmacol. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Gu, L.; Faig, A.; Abdelhamid, D.; Uhrich, K. Sugar-based amphiphilic polymers for biomedical applications: From nanocarriers to therapeutics. Acc. Chem. Res. 2014, 47, 2867–2877. [Google Scholar] [CrossRef]
- Yang, H.Y.; Jang, M.S.; Gao, G.H.; Lee, J.H.; Lee, D.S. pH-Responsive biodegradable polymeric micelles with anchors to interface magnetic nanoparticles for MR imaging in detection of cerebral ischemic area. Nanoscale 2016, 8, 12588–12598. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, A.; Cabana-Montenegro, S.; Aswal, V.K.; Lage, E.V.; Sandez-Macho, I.; Concheiro, A.; Alvarez-Lorenzo, C.; Bahadur, P. NaCl-triggered self-assembly of hydrophilic poloxamine block copolymers. Int. J. Pharm. 2015, 494, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiong, D.; Sun, Y.; Zhao, B.; Lin, W.; Zhang, L. Self-assembled micelles based on pH-sensitive PAE-g-MPEG-cholesterol block copolymer for anticancer drug delivery. Int. J. Nanomed. 2014, 9, 4923–4933. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Wang, Y.; Wang, X.; Wang, Q.; Chen, M. Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnol. Adv. 2014, 32, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, J.; Li, X.; Zhao, D.; Sun, J.; Liu, X. Self-assembly and emulsification of dopamine-modified hyaluronan. Carbohydr. Polym. 2015, 123, 72–79. [Google Scholar] [CrossRef] [PubMed]
- WhanLee, J.; HanPark, J.; Robinson, J.R. Bioadhesive-Based Dosage Forms: The Next Generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar]
- Wang, L.; Liu, Z.; Liu, D.; Liu, C.; Juan, Z.; Zhang, N. Docetaxel-loaded-lipid-based-nanosuspensions (DTX-LNS): Preparation, pharmacokinetics, tissue distribution and antitumor activity. Int. J. Pharm. 2011, 413, 194–201. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharyya, M.; Ernsting, M.J.; PMay, J.; Li, S.-D. Recent progress in the development of polysaccharide conjugates of docetaxel and paclitaxel. WIREs Nanomed. Nanobiotechnol. 2014, 6, 349–368. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Cheng, L.; He, Y.; Wei, X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: A review. Int. J. Biol. Macromol. 2018, 120, 2076–2085. [Google Scholar] [CrossRef]
- Guan, Q.; Zhang, G.; Sun, D.; Wang, Y.; Liu, K.; Wang, M.; Sun, C.; Li, B.; Zhang, Z.; Lv, J. In vitro and in vivo evaluation of docetaxel-loaded stearic acid-modified Bletilla striata polysaccharide copolymer micelles. PLoS ONE 2017, 12, e0173172. [Google Scholar] [CrossRef]
- Guan, Q.; Sun, D.; Zhang, G.; Sun, C.; Wang, M.; Ji, D.; Yang, W. Docetaxel-loaded self-assembly stearic acid-modified bletilla striata polysaccharide micelles and their anticancer effect: Preparation, characterization, cellular uptake and in vitro evaluation. Molecules 2016, 21, 1641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, D.; Lu, H.; Han, B.; Zhang, G.; Guan, Q. In vitro characterization of pH-sensitive Bletilla Striata polysaccharide copolymer micelles and enhanced tumour suppression in vivo. J. Pharm. Pharmacol. 2018, 70, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Z.; Chen, L.; Gu, W.; Li, Y. Chitosan N-betainates/DNA self-assembly nanoparticles for gene delivery: In vitro uptake and transfection efficiency. Int. J. Pharm. 2009, 371, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, H.; Zhai, G. Self-assembled nanoparticles based on chondroitin sulfate-deoxycholic acid conjugates for docetaxel delivery: Effect of degree of substitution of deoxycholic acid. Colloids Surf. B 2016, 146, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Park, J.H.; Chung, H.; Kwon, I.C.; Jeong, S.Y. Physicochemical characteristics of self-assembled nanoparticles based on glycol chitosan bearing 5β-cholanic acid. Langmuir 2003, 19, 10188–10193. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Surface Tailoring for Controlled Protein Adsorption Effect of Topography at the Nanometer Scale and Chemistry. J. Am. Chem. Soc. 2006, 128, 3939–3945. [Google Scholar] [CrossRef] [PubMed]
- Asuri, P.; Bale, S.S.; Karajanagi, S.S.; Kane, R.S. The protein-nanomaterial interface. Curr. Opin. Biotechnol. 2006, 17, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.S.; Ilanchelian, M. Comprehensive Multispectroscopic Analysis on the Interaction and Corona Formation of Human Serum Albumin with Gold/Silver Alloy Nanoparticles. J. Phys. Chem. B 2015, 119, 9461–9476. [Google Scholar] [CrossRef]
- Chakraborti, S.; Joshi, P.; Chakravarty, D.; Shanker, V.; Ansari, Z.A.; Singh, S.P.; Chakrabarti, P. Interaction of polyethyleneimine-functionalized ZnO nanoparticles with bovine serum albumin. Langmuir 2012, 28, 11142–11152. [Google Scholar] [CrossRef]
- Husain, M.A.; Ishqi, H.M.; Rehman, S.U.; Sarwar, T.; Afrin, S.; Rahman, Y.; Tabish, M. Elucidating the interaction of sulindac with calf thymus DNA: Biophysical and in silico molecular modelling approach. New J. Chem. 2017, 41, 14924–14935. [Google Scholar] [CrossRef]
- He, X.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chen, M.; Yang, W.; Zhou, Z.; Liu, L.; Zhang, Q. Interaction of bovine serum albumin with self-assembled nanoparticles of 6-O-cholesterol modified chitosan. Colloids Surf. B 2012, 92, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Raddam, Q.N.; Zeidan, M.M.; Abdulrahman, M.A.; K.Asaad, N. Smoking Effects on Blood Antioxidants Level: Lactate Dehydrogenase, Catalase, Superoxide Dismutase and Glutathione Peroxidase in University Students. J. Clin. Exp. 2017, 7, 1000331. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharma, G.; Sonali; Singh, S.; Patne, S.C.U.; Pandey, B.L.; Koch, B.; Muthu, M.S. Effects of transferrin conjugated multi-walled carbon nanotubes in lung cancer delivery. Mater. Sci. Eng. C 2016, 67, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-H.; Nguyen, C.T.; Gonzalez-Fajardo, L.; Hargrove, D.; Song, D.; Deshmukh, P.; Mahajan, L.; Ndaya, D.; Lai, L.; Kasi, R.M.; et al. Long circulating self-assembled nanoparticles from cholesterol-containing brush-like block copolymers for improved drug delivery to tumors. Biomacromolecules 2014, 15, 4363–4375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jackson, J.K.; M.Burt, H. Development of amphiphilic diblock copolymers as micellar carriers of taxol. Int. J. Pharm. 1996, 132, 195–206. [Google Scholar] [CrossRef]
- Shi, X.; Du, Y.; Yang, J.; Zhang, B.; Sun, L. Effect of degree of substitution and molecular weight of carboxymethyl chitosan nanoparticles on doxorubicin delivery. J. Appl. Polym. Sci. 2006, 100, 4689–4696. [Google Scholar] [CrossRef]
- Wong, H.; Bendayan, R.; Rauth, A.M.; Xue, H.; Babakhanian, K.; Wu, X. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J. Pharmacol. Exp. Ther. 2006, 317, 1372–1381. [Google Scholar] [CrossRef]
- Wong, H.; Rauth, A.M.; Bendayan, R.; Manias, J.L.; Ramaswamy, M.; Liu, Z.; Erhan, S.Z.; Wu, X.Y. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 2006, 23, 1574–1585. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Han, F. The retrospective analysis of rhizoma bletillae used as an antitumor medicine. Inf. Tradit. Chin. Med. 2013, 30, 148–150. [Google Scholar]
- Liu, H.; Tu, L.; Zhou, Y.; Dang, Z.; Wang, L.; Du, J.; Feng, J.; Hu, K. Improved Bioavailability and Antitumor Effect of Docetaxel by TPGS Modified Proniosomes: In Vitro and In Vivo Evaluations. Sci. Rep. 2017, 7, 43372. [Google Scholar] [CrossRef] [Green Version]
- Sardão, V.A.; Oliveira, P.J.; Holy, J.; Oliveira, C.R.; Wallace, K.B. Morphological alterations induced by doxorubicin on H9c2 myoblasts: Nuclear, mitochondrial, and cytoskeletal targets. Cell Biol. Toxicol. 2009, 25, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J. Cell. Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, R.; Chi, Z.; Teng, Y.; Qin, P. New Insights into the Behavior of Bovine Serum Albumin Adsorbed onto Carbon Nanotubes Comprehensive Spectroscopic Studies. J. Phys. Chem. B 2010, 114, 5626–5631. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.R.; Mohamad, S.B.; Bujang, N.; Malek, S.N.A.; Tayyab, S. Multispectroscopic and molecular modeling approach to investigate the interaction of flavokawain B with human serum albumin. J. Agric. Food. Chem. 2012, 60, 5899–5908. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Geng, F.; Liu, Y.; Wang, X.; Jiao, J.; Yu, L. Biomimetic interactions of proteins with functionalized cadmium sulfide quantum dots. Colloids Surf. A 2011, 392, 191–197. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, C.; Jiang, M.; Zhu, Y.; Wang, J.; Chen, J.; Shi, J. Binding interaction of atorvastatin with bovine serum albumin: Spectroscopic methods and molecular docking. Spectrochim. Acta Part A 2016, 156, 155–163. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, A.; Yuan, L. Interactions between O-carboxymethylchitosan and bovine serum albumin. Mater. Chem. Phys. 2008, 112, 41–46. [Google Scholar] [CrossRef]
- Lundqvist, M.; Sethson, I.; Jonsson, B.-H. Protein Adsorption onto Silica Nanoparticles: Conformational Changes Depend on the Particles’ Curvature and the Protein Stability. Langmuir 2004, 20, 10639–10647. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Fan, Y.; Ma, J. Interactions of some modified mono- and bis-beta-cyclodextrins with bovine serum albumin. Bioorg. Med. Chem. 2006, 14, 131–137. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Yoshimasu, T.; Oura, S.; Ohta, F.; Hirai, Y.; Naito, K.; Nakamura, R.; Nishiguchi, H.; Hashimoto, S.; Kawago, M.; Okamura, Y. Epidermal growth factor receptor mutations are associated with docetaxel sensitivity in lung cancer. J. Thorac. Oncol. 2011, 6, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, D.; Chen, M.; Duan, C.; Dai, W.; Jia, L.; Zhao, W. Studies on pharmacokinetics and tissue distribution of oridonin nanosuspensions. Int. J. Pharm. 2008, 355, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ernsting, M.J.; Murakami, M.; Undzys, E.; Aman, A.; Press, B.; Li, S.-D. A docetaxel-carboxymethylc-ellulose nanoparticle outperforms the approved taxane nanoformulation, Abraxane, in mouse tumor models with significant control of metastases. J. Control. Release 2012, 162, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, Q.; Chen, T.; Wu, W.; Lang, K.; Li, Z.; Li, J. Controlled co-delivery nanocarriers based on mixed micelles formed from cyclodextrin-conjugated and cross-linked copolymers. Colloids Surf. B 2014, 123, 486–492. [Google Scholar] [CrossRef] [PubMed]














| Parameters | Duopafei® | DTX-Loaded BSPs-SA Nanoparticles |
|---|---|---|
| t1/2 (h) | 0.83 ± 0.05 | 1.13 ± 0.06* |
| CL (L/h/kg) | 1.16 ± 0.06 | 0.82 ± 0.03* |
| MRT0–∞ (h) | 0.81 ± 0.06 | 1.37 ± 0.12* |
| AUC0–6 h (h mg/L) | 21.41 ± 1.04 | 29.38 ± 0.91* |
| AUC0–∞ (h mg/L) | 21.60 ± 1.07 | 30.72 ± 1.22* |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Qiao, J.; Liu, X.; Liu, Y.; Wu, J.; Huang, L.; Ji, D.; Guan, Q. Interactions of Self-Assembled Bletilla Striata Polysaccharide Nanoparticles with Bovine Serum Albumin and Biodistribution of Its Docetaxel-Loaded Nanoparticles. Pharmaceutics 2019, 11, 43. https://doi.org/10.3390/pharmaceutics11010043
Zhang G, Qiao J, Liu X, Liu Y, Wu J, Huang L, Ji D, Guan Q. Interactions of Self-Assembled Bletilla Striata Polysaccharide Nanoparticles with Bovine Serum Albumin and Biodistribution of Its Docetaxel-Loaded Nanoparticles. Pharmaceutics. 2019; 11(1):43. https://doi.org/10.3390/pharmaceutics11010043
Chicago/Turabian StyleZhang, Guangyuan, Jin Qiao, Xin Liu, Yuran Liu, Ji Wu, Long Huang, Danyang Ji, and Qingxiang Guan. 2019. "Interactions of Self-Assembled Bletilla Striata Polysaccharide Nanoparticles with Bovine Serum Albumin and Biodistribution of Its Docetaxel-Loaded Nanoparticles" Pharmaceutics 11, no. 1: 43. https://doi.org/10.3390/pharmaceutics11010043
APA StyleZhang, G., Qiao, J., Liu, X., Liu, Y., Wu, J., Huang, L., Ji, D., & Guan, Q. (2019). Interactions of Self-Assembled Bletilla Striata Polysaccharide Nanoparticles with Bovine Serum Albumin and Biodistribution of Its Docetaxel-Loaded Nanoparticles. Pharmaceutics, 11(1), 43. https://doi.org/10.3390/pharmaceutics11010043