Hyperthermia-Triggered Doxorubicin Release from Polymer-Coated Magnetic Nanorods
Abstract
:1. Background
2. Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Magnetic Nanorods
2.2.2. Surface Functionalization of MNRs
2.2.3. Morphology and Size Distribution
2.2.4. Electrophoretic Mobility
2.2.5. X-Ray Diffraction
2.2.6. Fourier Transform (FTIR) Infrared Characterization
2.2.7. Thermogravimetric Analysis
2.2.8. Magnetic Hyperthermia (MH) Response
2.2.9. Doxorubicin Loading and Release
3. Results and Discussion
3.1. Morphology and Particle Size Distribution
3.2. Electrical Characterization: Isoelectric Point Determination, Polymer Coating and Its Stability
3.3. XRD Diffraction
3.4. Thermogravimetric Analysis
3.5. ATR-FTIR Characterization
3.6. Magnetic Hyperthermia
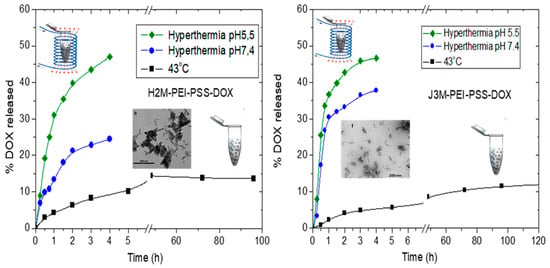
3.7. Doxorubicin Loading and Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. 2007, 80A, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, A.; Tombacz, E.; Illes, E.; Bica, D.; Vekas, L. Magnetite Nanoparticles Stabilized Under Physiological Conditions for Biomedical Application. In Colloids for Nano- and Biotechnology; Hórvölgyi, Z.D., Kiss, É., Eds.; Springer: Berlin, Germany, 2008; pp. 29–37. [Google Scholar]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Szalai, A.J.; Manivannan, N.; Kaptay, G. Super-paramagnetic magnetite nanoparticles obtained by different synthesis and separation methods stabilized by biocompatible coatings. Colloid. Surf. A Physicochem. Eng. Aspects 2019, 568, 113–122. [Google Scholar] [CrossRef]
- Delgado, A.V.; López-Viota, J.; Ramos-Tejada, M.M.; Arias, J.L. Particle geometry, charge, and wettability: The fate of nanoparticle-based drug vehicles. In Colloid and Interface Science in Pharmaceutical Research and Development; Ohshima, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 443–467. [Google Scholar]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.D.; Jay, M.; Dziublal, T.D.; Lu, X. PEGylation of nanocarrier drug delivery systems: State of the art. J. Biomed. Nanotechnol. 2008, 4, 133–148. [Google Scholar] [CrossRef]
- Hong, R.Y.; Feng, B.; Chen, L.L.; Liu, G.H.; Li, H.Z.; Zheng, Y.; Wei, D.G. Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Eng. J. 2008, 42, 290–300. [Google Scholar] [CrossRef]
- Arias, J.L.; Reddy, L.H.; Couvreur, P. Fe3O4/chitosan nanocomposite for magnetic drug targeting to cancer. J. Mater. Chem. 2012, 22, 7622–7632. [Google Scholar] [CrossRef]
- Richardson, P.F. Nanotechnology Therapeutics in Oncology-Recent Developments and Future Outlook. Annu. Rep. Med. Chem. 2012, 47, 239–252. [Google Scholar]
- Han, C.P.; Zhang, A.W.; Kong, Y.; Yu, N.N.; Xie, T.; Dou, B.R.; Li, K.; Wang, Y.; Li, J.; Xu, K. Multifunctional iron oxide-carbon hybrid nanoparticles for targeted fluorescent/MR dual-modal imaging and detection of breast cancer cells. Anal. Chim. Acta 2019, 1067, 115–128. [Google Scholar] [CrossRef]
- Loomis, K.; McNeeley, K.; Bellamkonda, R.V. Nanoparticles with targeting, triggered release, and imaging functionality for cancer applications. Soft. Matter. 2011, 7, 839–856. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Hosseini, M. Smart Polymeric-Based Microencapsulation: A Promising Synergic Combination; Industrial Applications for Intelligent Polymers and Coatings; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Yazdani, F.; Seddigh, M. Magnetite nanoparticles synthesized by co-precipitation method: The effects of various iron anions on specifications. Mater. Chem. Phys. 2016, 184, 318–323. [Google Scholar] [CrossRef]
- Lemine, O.M.; Omri, K.; Zhang, B.; El Mir, L.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol-gel synthesis of 8 nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattices Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, A.V.; Schneider, E.K.; Fernandez, B.L.C.; Iglesias, G.R. Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties. Polymers 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. PNAS 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [Green Version]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Rel. 2007, 121, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Hwang, D.K.; Dendukuri, D.; Doyle, P.S. Microfluidic-based synthesis of non-spherical magnetic hydrogel microparticles. Lab. A Chip 2008, 8, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Mohammad, F. Magnetic Nanomaterials for Hyperthermia-based Therapy and Controlled Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Kobayashi, T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol. J. 2011, 6, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I. In vivo applications of magnetic nanoparticle hyperthermia. Int. J. Hyperth. 2013, 29, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Mertz, D.; Sandre, O.; Begin-Colin, S. Drug releasing nanoplatforms activated by alternating magnetic fields. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1617–1641. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Li, M.H.; Bu, W.B.; Ren, J.; Li, J.B.; Deng, L.; Gao, M.Y.; Gao, X.; Wang, P. Enhanced Synergism of Thermo-chemotherapy for Liver Cancer with Magnetothermally Responsive Nanocarriers. Theranostics 2018, 8, 693–709. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, P.; Liu, C.H.; Zhi, X.T. Doxorubicin-loaded mesoporous magnetic nanoparticles to induce apoptosis in breast cancer cells. Biomed. Pharmacother. 2015, 69, 355–360. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Kim, D.W.; Jones, E.; Lan, L.; McCall, L.; Dewhirst, M.W.; Craciunescu, O.; Stauffer, P.; Liotcheva, V.; Betof, A.; et al. A phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int. J. Hyperth. 2010, 26, 514–521. [Google Scholar] [CrossRef]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 2017, 43, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, B.P.; Raghunand, N.; Baggett, B.; Gillies, R.J. Tumor acidity, ion trapping and chemotherapeutics I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem. Pharm. 2003, 66, 1207–1218. [Google Scholar] [CrossRef]
- Reyes-Ortega, F. pH-responsive polymers: Properties, synthesis and applications. In Smart Polymers and their Applications; Aguilar, M.R., San Román, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Huang, W.J.; Yang, F.C.; Zhu, L.; Qiao, R.; Zhao, Y.P. Manipulation of magnetic nanorod clusters in liquid by non-uniform alternating magnetic fields. Soft. Matter. 2017, 13, 3750–3759. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, G.; Delgado, A.V.; Kujda, M.; Ramos-Tejada, M.M. Magnetic hyperthermia with magnetite nanoparticles: Electrostatic and polymeric stabilization. Coll. Polym. Sci. 2016, 294, 1541–1550. [Google Scholar] [CrossRef]
- Iglesias, G.R.; Reyes-Ortega, F.; Fernandez, B.L.C.; Delgado, A.V. Hyperthermia-Triggered Gemcitabine Release from Polymer-Coated Magnetite Nanoparticles. Polymers 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Ocaña, M.M.; Morales, M.P.; Serna, C.J. The growth-mechanism of α-Fe2O3 ellipsoidal particles in solution. J. Colloid Interface Sci. 1995, 171, 85–91. [Google Scholar] [CrossRef]
- Mozo, S.L.; Zuddas, E.; Casu, A.; Falqui, A. Synthesizing iron oxide nanostructures: The polyetileneimine (PEI) role. Crystals 2017, 7, 22. [Google Scholar]
- Chen, X.Y.; Li, Y.X.; Huang, L.; Zou, D.; Wu, E.X.; Liu, Y.J.; Xie, Y.; Yao, R.; Liao, S.; Wang, G.; et al. Effects of Precipitant and pH on Coprecipitation of Nanosized Co-Cr-V Alloy Powders. Materials 2017, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.G.; Mukherjee, D.; Reddy, B.M. Novel approaches for preparation of nanoparticles. In Nanostructures for Novel Therapy: Synthesis, Characterization and Applications; Ficai, D., Grumezescu, A.M., Eds.; Elsevier: London, UK, 2017; pp. 1–36. [Google Scholar]
- Favela-Camacho, S.E.; Samaniego-Benitez, E.J.; Godinez-Garcia, A.; Aviles-Arellano, L.M.; Perez-Robles, J.F. How to decrease the agglomeration of magnetite nanoparticles and increase their stability using surface properties. Colloids Surf. A Phys. Eng. Asp. 2019, 574, 29–35. [Google Scholar] [CrossRef]
- Goldt, A.E.; Polyakov, A.Y.; Sorkina, T.A.; Dubov, A.L.; Davidova, G.A.; Selezneva, I.I.; Maximov, Y.V.; Presnyakov, N.; Polyakova, Y.; Goodilin, E.A.; et al. Humic acid-stabilized superparamagnetic maghemite nanoparticles: Surface charge and embryotoxicity evaluation. Nanosyst. Phys. Chem. Math. 2019, 10, 184–189. [Google Scholar] [CrossRef]
- Rikukawa, M.; Sanui, K. Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog. Polym. Sci. 2000, 25, 1463–1502. [Google Scholar] [CrossRef]
- Huang, Y.P.; Lin, I.J.; Chen, C.C.; Hsu, Y.C.; Chang, C.C.; Lee, M.J. Delivery of small interfering RNAs in human cervical cancer cells by polyethylenimine-functionalized carbon nanotubes. Nanoscale Res. Lett. 2013, 8, 267. [Google Scholar]
- Qu, Y.X.; Yang, Y.X.; Zou, Z.S.; Zeilstra, C.; Meijer, K.; Boom, R. Thermal Decomposition Behaviour of Fine Iron Ore Particles. ISIJ Int. 2014, 54, 2196–2205. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estadé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Martinez, L.M.; Khurshid, H.; Garaio, E.; Garcia, J.A.; Phan, M.H.; Srikanth, H. Enhanced Magnetic Hyperthermia in Iron Oxide Nano-Octopods: Size and Anisotropy Effects. J. Phys. Chem. C 2016, 120, 8370–8379. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Porshokouh, Z.N.; Kalappattil, V.; Torres, D.; Phan, M.H.; Garaio, E.; García, J.A.; Sanchez Llamazares, J.L.; Srikanth, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Ludwig, R.; Stapf, M.; Dutz, S.; Muller, R.; Teichgraber, U.; Hilger, I. Structural properties of magnetic nanoparticles determine their heating behavior—An estimation of the in vivo heating potential. Nanoscale Res. Lett. 2014, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Nikitin, A.; Khramtsov, M.; Garanina, A.; Mogilnikov, P.; Sviridenkova, N.; Shchetinin, I.; Savchenko, A.; Abakumov, M.; Majouga, A. Synthesis of iron oxide nanorods for enhanced magnetic hyperthermia. J. Magn. Magn. Mater. 2019, 469, 443–449. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Wong, L.H.; Schumann, C.; Sabei, F.Y.; Korzun, T.; Li, X.; Hansen, M.N.; Dhagat, P.; Moses, A.S.; Taratula, O.; et al. Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia. ACS Nano 2019, 13, 6383–6395. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.H.; Na, W.; Jang, J.T.; Lee, J.H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, W.S.; Kim, J.H. Surface modification of functional nanoparticles for controlled drug delivery. J. Dispers. Sci. Technol. 2003, 24, 475–487. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control Rel. 2010, 148, 135–146. [Google Scholar] [CrossRef]






| Sample | Electrophoretic Mobility (10−8 ms−1/Vm−1) |
|---|---|
| H2M | −2.23 ± 0.08 |
| H2M-PEI | 2.10 ± 0.04 |
| H2M-PEI-PSS | −3.73 ± 0.13 |
| H2M-PEI-PSS-DOX | 0.79 ± 0.05 |
| J3M | −2.48 ± 0.08 |
| J3M-PEI | 0.10 ± 0.05 |
| J3M-PEI-PSS | −4.8 ± 0.3 |
| J3M-PEI-PSS-DOX | −3.5 ± 0.4 |
| H2M-PEI-PSS | % Weight | J3M-PEI-PSS | % Weight |
|---|---|---|---|
| Fe3O4 | 89.6 | γ-Fe2O3 | 92.0 |
| PEI | 5.1 | PEI | 3.2 |
| PSS | 5.3 | PSS | 4.8 |
| Sample | SAR (W/g) | ILP (nHm2/kg) |
|---|---|---|
| H2M | 26–43 | 1.12 ± 0.06 |
| H2M-PEI-PSS | 6–13 | 0.24 ± 0.04 |
| J3M | 15–26 | 0.52 ± 0.08 |
| J3M-PEI-PSS | 10–25 | 0.29 ± 0.05 |
| Sample | % Adsorbed DOX | µg DOX/mg MNRs |
|---|---|---|
| H2M-PEI-PSS | 64.4 | 7.0 |
| J3M-PEI-PSS | 84.1 | 20.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Ortega, F.; Checa Fernández, B.L.; Delgado, A.V.; Iglesias, G.R. Hyperthermia-Triggered Doxorubicin Release from Polymer-Coated Magnetic Nanorods. Pharmaceutics 2019, 11, 517. https://doi.org/10.3390/pharmaceutics11100517
Reyes-Ortega F, Checa Fernández BL, Delgado AV, Iglesias GR. Hyperthermia-Triggered Doxorubicin Release from Polymer-Coated Magnetic Nanorods. Pharmaceutics. 2019; 11(10):517. https://doi.org/10.3390/pharmaceutics11100517
Chicago/Turabian StyleReyes-Ortega, Felisa, Blanca Luna Checa Fernández, Angel V. Delgado, and Guillermo R. Iglesias. 2019. "Hyperthermia-Triggered Doxorubicin Release from Polymer-Coated Magnetic Nanorods" Pharmaceutics 11, no. 10: 517. https://doi.org/10.3390/pharmaceutics11100517
APA StyleReyes-Ortega, F., Checa Fernández, B. L., Delgado, A. V., & Iglesias, G. R. (2019). Hyperthermia-Triggered Doxorubicin Release from Polymer-Coated Magnetic Nanorods. Pharmaceutics, 11(10), 517. https://doi.org/10.3390/pharmaceutics11100517