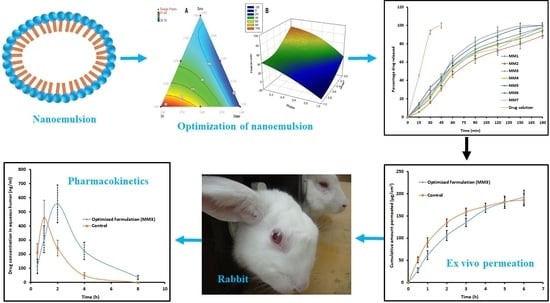
Nanoemulsion Based Vehicle for Effective Ocular Delivery of Moxifloxacin Using Experimental Design and Pharmacokinetic Study in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drug Analysis
2.3. Development of Pseudoternary Phase Diagram
2.4. Preparation of Moxifloxacin Nanoemulsion
2.5. Experimental Design
2.6. Characterization of Moxifloxacin Nanoemulsions
2.6.1. Drug Content and pH
2.6.2. Transmittance, Conductivity and Dilution Potential
2.6.3. Particle Size Characterization and Zeta Potential
2.6.4. Viscosity
2.7. Transmission Electron Microscopy (TEM)
2.8. In Vitro Release
- Zero order model Q = Q0 + kt
- First order model Q = Q0 × ekt
- Higuchi model Q = k × t0.5
- Hixson-Crowell model Q1/3 = kt + Q01/3
- Korsmeyer–Peppas model Q = k × tn
- Weibull model Q = 1 − exp[−(t)b/a]
2.9. Ex Vivo Permeation
2.10. Antimicrobial Efficacy
2.11. Ocular Irritation
2.12. Pharmacokinetics
2.13. Stability
2.14. Data Analysis
3. Results and Discussion
3.1. Pseudoternary Phase Diagram
3.2. Formulation Optimization
3.3. Validation of Applied Design
3.4. Characterization of Nanoemulsion
3.5. TEM
3.6. In Vitro Release
3.7. Ex Vivo Permeation
3.8. Antimicrobial Efficacy
3.9. Ocular Irritation
3.10. Pharmacokinetics in the Aqueous Humor
3.11. Stability Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; De La Fuente, M. Nanotherapies for the treatment of ocular diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Alani, A.W.; Alany, R.G. Recent advances in non-ionic surfactant vesicles (niosomes): Self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Deliv. 2014, 21, 87–100. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Abdul Nasir, N.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2016, 23, 1075–1091. [Google Scholar] [CrossRef]
- Ali, A.; Ansari, V.A.; Ahmad, U.; Akhtar, J.; Jahan, A. Nanoemulsion: An advanced vehicle for efficient drug delivery. Drug Res. 2017, 67, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Alany, R.G.; Rades, T.; Nicoll, J.; Tucker, I.G. Davies NM. W/O microemulsions for ocular delivery: Evaluation of ocular irritation and precorneal retention. J. Control. Release 2006, 111, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Miller, D. Review of moxifloxacin hydrochloride ophthalmic solution in the treatment of bacterial eye infections. Clin. Ophthalmol. 2008, 2, 77–91. [Google Scholar] [CrossRef]
- Sultana, N.; Arayne, M.S.; Akhtar, M.; Shamim, S.; Gul, S.; Khan, M.M. High-performance liquid chromatography assay for moxifloxacin in bulk, pharmaceutical formulations and serum: Application to in-vitro metal interactions. J. Chin. Chem. Soc. 2010, 57, 708–717. [Google Scholar] [CrossRef]
- Ma, Y.J.; Yuan, X.Z.; Huang, H.J.; Xiao, Z.H.; Zeng, G.M. The pseudo-ternary phase diagrams and properties of anionic–nonionic mixed surfactant reverse micellar systems. J. Mol. Liq. 2015, 203, 181–186. [Google Scholar] [CrossRef]
- Syed, H.K.; Peh, K.K. Identification of phases of various oil, surfactant/co-surfactants and water system by ternary phase diagram. Acta Pol. Pharm. 2014, 71, 301–309. [Google Scholar] [PubMed]
- Fialho, S.L.; Da Silva-Cunha, A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016, 23, 2154–2162. [Google Scholar] [CrossRef]
- Morsy, M.A.; Nair, A.B. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. Int. J. Pharm. 2018, 552, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.N.; Patel, R.K.; Shah, H.J.; Mehta, T.A. Beyond the blink: Using in-situ gelling to optimize ophthalmic drug delivery. Pharm. Technol. 2015, 39, 1–7. [Google Scholar]
- Shah, H.; Nair, A.B.; Shah, J.; Bharadia, P.; Al-Dhubiab, B.E. Proniosomal gel for transdermal delivery of lornoxicam: Optimization using factorial design and in vivo evaluation in rats. DARU J. Pharm. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Formulation and evaluation of nano based drug delivery system for the buccal delivery of acyclovir. Colloids Surf. B Biointerfaces 2015, 136, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf. B Biointerfaces 2010, 81, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Gan, Y.; Zhu, C.; Zhang, X.; Zhu, J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: In vitro and in vivo results. Int. J. Pharm. 2009, 365, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Al-Dhubiab, B.E. Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition. J. Liposome Res. 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Kumria, R.; Al-Dhubiab, B.E.; Shah, J.; Nair, A.B. Formulation and evaluation of chitosan-based buccal bioadhesive films of zolmitriptan. J. Pharm. Innov. 2018, 13, 133–143. [Google Scholar] [CrossRef]
- Wadhwa, J.; Nair, A.; Kumria, R. Self-emulsifying therapeutic system: A potential approach for delivery of lipophilic drugs. Braz. J. Pharm. Sci. 2011, 47, 447–465. [Google Scholar] [CrossRef]
- Hegde, R.R.; Verma, A.; Ghosh, A. Microemulsion: New insights into the ocular drug delivery. ISRN Pharm. 2013, 2013, 826798. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.F.; Zheng, L.Q.; Tung, C.H. Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system. Int. J. Pharm. 2005, 301, 237–246. [Google Scholar] [CrossRef]
- Shah, S.M.; Jain, A.S.; Kaushik, R.; Nagarsenker, M.S.; Nerurkar, M.J. Preclinical formulations: Insight, strategies, and practical considerations. AAPS PharmSciTech. 2014, 15, 1307–1323. [Google Scholar] [CrossRef]
- Schneider, K.; Ott, T.M.; Schweins, R.; Frielinghaus, H.; Lade, O.; Sottmann, T. Phase behavior and microstructure of symmetric nonionic microemulsions with long-chain n-alkanes and waxes. Ind. Eng. Chem. Res. 2019, 58, 2583–2595. [Google Scholar] [CrossRef]
- Tiffany, J.M. The viscosity of human tears. Int. Ophthalmol. 1991, 15, 371–376. [Google Scholar] [CrossRef]
- Robertson, S.M.; Curtis, M.A.; Schlech, B.A.; Rusinko, A.; Owen, G.R.; Dembinska, O.; Liao, J.; Dahlin, D.C. Ocular pharmacokinetics of moxifloxacin after topical treatment of animals and humans. Surv. Ophthalmol. 2005, 50, S32–S45. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Dembry, L.M.; Farrel, P.A.; Callan, D.A.; Andriole, V.T. Comparative antimicrobial activity of gatifloxacin with ciprofloxacin and beta-lactams against gram-positive bacteria. Diagn. Microbiol. Infect. Dis. 2001, 41, 143–148. [Google Scholar] [CrossRef]
- Nair, A.B.; Kaushik, A.; Attimarad, M.; Al-Dhubiab, B.E. Enhanced oral bioavailability of calcium using bovine serum albumin microspheres. Drug Deliv. 2012, 19, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y. Development of an ex vivo method for evaluation of precorneal residence of topical ophthalmic formulations. AAPS PharmSciTech. 2009, 10, 796–805. [Google Scholar] [CrossRef]






| Formulations | Run | Formulation Components | Transformed Proportion | ||||
|---|---|---|---|---|---|---|---|
| Smix (%) | Oil (%) | Water (%) | Smix | Oil | Water | ||
| MM1 | 1 | 52 | 4 | 44 | 1 | 0 | 0 |
| MM2 | 2 | 36 | 20 | 44 | 0 | 1 | 0 |
| MM3 | 3 | 36 | 4 | 60 | 0 | 0 | 1 |
| MM4 | 4 | 44 | 12 | 44 | 0.5 | 0.5 | 0 |
| MM5 | 5 | 44 | 4 | 52 | 0.5 | 0 | 0.5 |
| MM6 | 6 | 36 | 12 | 52 | 0 | 0.5 | 0.5 |
| MM7 | 7 | 41.33 | 9.33 | 49.33 | 0.33 | 0.33 | 0.33 |
| MM8 * | 8 * | 37 | 18 | 45 | 0.063 | 0.875 | 0.063 |
| Parameter | MM1 | MM2 | MM3 | MM4 | MM5 | MM6 | MM7 | MM8 ** |
|---|---|---|---|---|---|---|---|---|
| Drug content (%) | 96.05 ± 4.01 | 93.38 ± 2.92 | 99.90 ± 2.62 | 94.47 ± 3.48 | 101.62 ± 2.29 | 102.06 ± 2.47 | 98.25 ± 3.78 | 95.34 ± 4.62 |
| pH | 6.33 ± 0.41 | 6.70 ± 0.52 | 6.64 ± 0.43 | 6.85 ± 0.32 | 6.19 ± 0.28 | 6.22 ± 0.44 | 7.04 ± 0.54 | 6.51 ± 0.36 |
| Transmittance (%) | 97.22 ± 5.72 | 95.38 ± 5.29 | 97.82 ± 3.83 | 96.17 ± 3.92 | 97.71 ± 3.94 | 95.46 ± 4.42 | 96.43 ± 4.85 | 97.62 ± 3.63 |
| Conductivity (mS/cm) | 0.11 ± 0.02 | 0.08 ± 0.01 | 0.20 ± 0.08 | 0.14 ± 0.04 | 0.15 ± 0.07 | 0.16 ± 0.06 | 0.14 ± 0.03 | 0.11 ± 0.03 |
| Dilution potential | >10 times | >10 times | >10 times | >10 times | >10 times | >10 times | >10 times | >10 times |
| Droplets size (nm) | 41.82 ± 13.71 | 81.04 ± 15.35 | 28.78 ± 10.34 | 47.56 ± 16.28 | 32.41 ± 14.70 | 67.15 ± 15.84 | 47.42 ± 14.11 | 75.99 ± 16.36 |
| Polydispersity index | 0.35 ± 0.05 | 0.26 ± 0.03 | 0.38 ± 0.06 | 0.39 ± 0.04 | 0.34 ± 0.02 | 0.30 ± 0.05 | 0.39 ± 0.03 | 0.24 ± 0.02 |
| Zeta potential (mV) | −0.33 ± 0.01 | −0.35 ± 0.02 | −0.38 ± 0.012 | −0.28 ± 0.02 | −0.32 ± 0.03 | 0.37 ± 0.03 | −0.29 ± 0.02 | −0.32 ± 0.01 |
| Viscosity (cP) | 4.81 ± 1.67 | 6.50 ± 1.17 | 3.28 ± 1.42 | 5.80 ± 1.37 | 4.57 ± 1.47 | 5.86 ± 2.44 | 4.92 ± 1.85 | 6.39 ± 2.24 |
| Model Name | Multiple R | R Square | X Variable | Slope | SSR | Fischer Ratio |
|---|---|---|---|---|---|---|
| Zero order | 0.9486 | 0.8999 | 0.5862 | 12.7455 | 1194.4235 | 170.6319 |
| First order | 0.9558 | 0.9135 | −0.0166 | 2.3706 | 20804.4358 | 2972.0623 |
| Higuchi | 0.9740 | 0.9486 | 8.7765 | −10.4853 | 613.1492 | 87.5927 |
| Korsmeyer–Peppas | 0.9766 | 0.9538 | 0.8446 | −1.8055 | 1086.6082 | 155.2297 |
| Weibull Model | 0.9904 | 0.9810 | 1.6232 | −2.8693 | 4932.8215 | 704.6888 |
| Hixson–Crowell | 0.9940 | 0.9880 | 0.0271 | −0.2801 | 5019.6483 | 717.0926 |
| Concentration (μg/mL) | Zone of Inhibition in (cm) | |
|---|---|---|
| Control * | MM3 | |
| Staphylococcus aureus | ||
| 1 | 1.63 ± 0.22 | 1.63 ± 0.26 |
| 10 | 2.44 ± 0.31 | 2.58 ± 0.18 |
| 100 | 3.52 ± 0.25 | 3.94 ± 0.24 |
| Pseudomonas aeruginosa | ||
| 1 | 1.82 ± 0.34 | 1.97 ± 0.16 |
| 10 | 2.77 ± 0.23 | 3.05 ± 0.21 |
| 100 | 4.05 ± 0.25 | 4.71 ± 0.29 |
| Parameter | Nanoemulsion (MM3) | Control |
|---|---|---|
| Tmax (h) | 2 | 1 |
| Cmax (ng/mL) | 555.73 ± 133.34 | 454.19 ± 126.91 |
| AUC0–8 (ng.h/mL) | 1859.76 ± 424.51 * | 958.63 ± 206.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H.; Shehata, T.M.; Morsy, M.A. Nanoemulsion Based Vehicle for Effective Ocular Delivery of Moxifloxacin Using Experimental Design and Pharmacokinetic Study in Rabbits. Pharmaceutics 2019, 11, 230. https://doi.org/10.3390/pharmaceutics11050230
Shah J, Nair AB, Jacob S, Patel RK, Shah H, Shehata TM, Morsy MA. Nanoemulsion Based Vehicle for Effective Ocular Delivery of Moxifloxacin Using Experimental Design and Pharmacokinetic Study in Rabbits. Pharmaceutics. 2019; 11(5):230. https://doi.org/10.3390/pharmaceutics11050230
Chicago/Turabian StyleShah, Jigar, Anroop B. Nair, Shery Jacob, Rakesh K. Patel, Hiral Shah, Tamer M. Shehata, and Mohamed Aly Morsy. 2019. "Nanoemulsion Based Vehicle for Effective Ocular Delivery of Moxifloxacin Using Experimental Design and Pharmacokinetic Study in Rabbits" Pharmaceutics 11, no. 5: 230. https://doi.org/10.3390/pharmaceutics11050230
APA StyleShah, J., Nair, A. B., Jacob, S., Patel, R. K., Shah, H., Shehata, T. M., & Morsy, M. A. (2019). Nanoemulsion Based Vehicle for Effective Ocular Delivery of Moxifloxacin Using Experimental Design and Pharmacokinetic Study in Rabbits. Pharmaceutics, 11(5), 230. https://doi.org/10.3390/pharmaceutics11050230