Development and In Vitro-In Vivo Evaluation of a Novel Sustained-Release Loxoprofen Pellet with Double Coating Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC-Assay for LXP and CA Contents
2.3. Solubility/pH Profiles of LXP
2.4. Preparation of Drug-Loaded Pellets
2.5. Preparation of Sustained-Release Pellets
2.5.1. Preparation of the Dissolution-Rate Controlling Layer
2.5.2. Preparation of the Diffusion-Rate Controlling Layer
2.6. Experimental Design
2.7. In Vitro Release of LXP and CA
2.8. Release Mechanism Studies
2.9. Morphology Study
2.10. The Pharmacokinetic Studies
2.10.1. Administration Programme
2.10.2. Determination of LXP in Plasma
2.10.3. Bioavailability Study
3. Results and Discussions
3.1. Impact of CA on Drug Release
3.2. Release Experiments and Statistical Evaluation
3.2.1. Testing of Drug Release
3.2.2. Regression Equations
3.2.3. Response Surface Plots
3.2.4. Design Space and Formulation Parameters Optimization
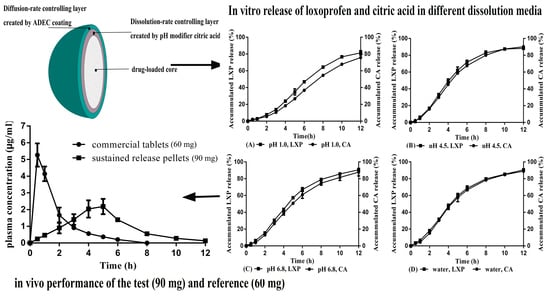
3.3. Simultaneous Release of CA and LXP from the Optimal Formulation in Different Dissolution Media
3.4. Release Mechanism Studies
3.5. Scanning Electron Photomicrographs
3.6. Pharmacokinetic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peter, J.C. Tramadol SR formulations: Pharmacokinetic comparison of a multiple-units dose (capsule) versus a single-unit dose (tablet). Clin. Drug Investig. 2005, 7, 435–443. [Google Scholar]
- Chen, T.; Li, J.; Chen, T.; Sun, C.C.; Zheng, Y. Tablets of multi-unit pellet system for controlled drug delivery. J. Control. Release 2017, 262, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A. Mechanisms of polymeric film formation. Int. J. Pharm. 2013, 457, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Howick, K.; Alam, R.; Chruscicka, B.; Kandil, D.; Fitzpatrick, D.; Ryan, A.M.; Cryan, J.F.; Schellekens, H.; Griffin, B.T. Sustained-release multiparticulates for oral delivery of a novel peptidic ghrelin agonist: Formulation design and in vitro characterization. Int. J. Pharm. 2018, 536, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Thapa, R.; Choi, D.H.; Jeong, S.H. Effects of pharmaceutical processes on the quality of ethylcellulose coated pellets: Quality by design approach. Powder Technol. 2018, 339, 25–38. [Google Scholar] [CrossRef]
- López, E.V.; Luzardo Álvarez, A.; Blanco Méndez, J.; Otero Espinar, F.J. Cellulose-polysaccharide film-coating of cyclodextrin based pellets for controlled drug release. J. Drug Deliv. Sci. Technol. 2017, 42, 273–283. [Google Scholar] [CrossRef]
- Dekyndt, B.; Verin, J.; Neut, C.; Siepmann, F.; Siepmann, J. How to easily provide zero order release of freely soluble drugs from coated pellets. Int. J. Pharm. 2015, 478, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Muschert, S.; Siepmann, F.; Leclercq, B.; Carlin, B.; Siepmann, J. Prediction of drug release from ethylcellulose coated pellets. J. Control. Release 2009, 135, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Ford, J.L.; Rajabi-Siahboomi, A. The influence of drug type on the release profiles from Surelease-coated pellets. Int. J. Pharm. 2003, 254, 123–135. [Google Scholar] [CrossRef]
- Terada, A.; Naruto, S.; Wachi, K.; Tanaka, S.; Iizuka, Y.; Misaka, E. Synthesis and antiinflammatory activity of [(cycloalkylmethyl)phenyl]acetic acids and related compounds. J. Med. Chem. 1984, 27, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Bao, C.D.; Chen, Z.W.; Zheng, Y.; Wang, G.C.; Zhao, D.B.; Hu, S.X.; Li, Y.J.; Shao, Z.W.; Zhang, Z.Y. Efficacy and safety of loxoprofen hydrogel patch versus loxoprofen tablet in patients with knee osteoarthritis: A randomized controlled non-inferiority trial. Clin. Rheumatol. 2016, 35, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Park, C.H.; Lee, Y.B. Direct and simultaneous analysis of loxoprofen and its diastereometric alcohol metabolites in human serum by on-line column switching liquid chromatography and its application to a pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 835, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, K.; Murakami, K.; Yamauchi, M.; Matsunari, O.; Ogawa, R.; Nakagawa, Y.; Okimoto, T.; Kodama, M.; Fujioka, T. Evaluation of selective cyclooxygenase-2 inhibitor-induced small bowel injury: Randomized cross-over study compared with loxoprofen in healthy subjects. Dig. Endosc. 2013, 25, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, N.; Suemasu, S.; Matoyama, M.; Kimoto, A.; Takeda, M.; Tanaka, K.; Ishihara, T.; Katsu, T.; Okamoto, Y.; Otsuka, M.; et al. Properties and synthesis of 2-{2-fluoro (or bromo)-4-[(2-oxocyclopentyl)methyl]phenyl}propanoic acid: Nonsteroidal anti-inflammatory drugs with low membrane permeabilizing and gastric lesion-producing activities. J. Med. Chem. 2010, 53, 7879–7882. [Google Scholar] [CrossRef]
- Tak, J.W.; Gupta, B.; Thapa, R.K.; Woo, K.B.; Kim, S.Y.; Go, T.G.; Choi, Y.; Choi, J.Y.; Jeong, J.H.; Choi, H.G.; et al. Preparation and optimization of immediate release/sustained release bilayered tablets of loxoprofen using box-behnken design. AAPS PharmSciTech 2017, 18, 1125–1134. [Google Scholar] [CrossRef]
- Venkatesan, P.; Manavalan, R.; Valliappan, K. Preparation and evaluation of sustained release loxoprofen loaded microspheres. J. Basic. Clin. Pharm. 2011, 2, 159–162. [Google Scholar]
- Zaman, M.; Rasool, S.; Ali, M.Y.; Qureshi, J.; Adnan, S.; Hanif, M.; Sarfraz, R.M.; Ijaz, H.; Mahmood, A. Fabrication and analysis of hydroxypropylmethyl cellulose and pectin-based controlled release matrix tablets loaded with loxoprofen sodium. Adv. Polym. Technol. 2015, 34, 1624–1631. [Google Scholar] [CrossRef]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly(AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef]
- Sonawane, R.O.; Patil, S.D. Fabrication and statistical optimization of starch-kappa-carrageenan cross-linked hydrogel composite for extended release pellets of zaltoprofen. Int. J. Biol. Macromol. 2018, 120, 2324–2334. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Upadhye, S.B.; Vladyka, R.S.; Repka, M.A. Formulation and development of pH-independent/dependent sustained release matrix tablets of ondansetron HCl by a continuous twin-screw melt granulation process. Int. J. Pharm. 2015, 496, 33–41. [Google Scholar] [CrossRef]
- Kasashima, Y.; Uchida, S.; Yoshihara, K.; Yasuji, T.; Sako, K.; Namiki, N. Oral sustained-release suspension based on a lauryl sulfate salt/complex. Int. J. Pharm. 2016, 515, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.H.; Nguyen, H.V.; Park, C.; Choi, Y.W.; Lee, B.J. Modulation of microenvironmental pH for dual release and reduced in vivo gastrointestinal bleeding of aceclofenac using hydroxypropyl methylcellulose-based bilayered matrix tablet. Eur. J. Pharm. Sci. 2017, 102, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ploen, J.; Andersch, J.; Heschel, M.; Leopold, C.S. Citric acid as a pH-modifying additive in an extended release pellet formulation containing a weakly basic drug. Drug Dev. Ind. Pharm. 2009, 35, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Corrigan, O.I. Predicting the dissolution rate of ibuprofen-acidic excipient compressed mixtures in reactive media. Int. J. Pharm. 1992, 84, 167–173. [Google Scholar] [CrossRef]
- Badawy, S.I.; Hussain, M.A. Microenvironmental pH modulation in solid dosage forms. J. Pharm. Sci. 2007, 96, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Luo, Y.; Feng, J.; Xu, M.; Tao, X.; He, H.; Tang, X. Preparation and in vitro–in vivo evaluation of none gastric resident dipyridamole (DIP) sustained-release pellets with enhanced bioavailability. Int. J. Pharm. 2012, 422, 9–16. [Google Scholar] [CrossRef]
- Frenning, G. Modelling drug release from inert matrix systems: From moving-boundary to continuous-field descriptions. Int. J. Pharm. 2011, 418, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuppok, Y.; Muschert, S.; Marucci, M.; Hjaertstam, J.; Siepmann, F.; Axelsson, A.; Siepmann, J. Drug release mechanisms from Kollicoat SR: Eudragit NE coated pellets. Int. J. Pharm. 2011, 409, 30–37. [Google Scholar] [CrossRef]
- Marucci, M.; Ragnarsson, G.; Nilsson, B.; Axelsson, A. Osmotic pumping release from ethyl-hydroxypropyl-cellulose-coated pellets: A new mechanistic model. J. Control. Release 2010, 142, 53–60. [Google Scholar] [CrossRef]
- Marucci, M.; Ragnarsson, G.; Axelsson, A. Evaluation of osmotic effects on coated pellets using a mechanistic model. Int. J. Pharm. 2007, 336, 67–74. [Google Scholar] [CrossRef]
- Ferrero, C.; Massuelle, D.; Doelker, E. Towards elucidation of the drug release mechanism from compressed hydrophilic matrices made of cellulose ethers. II. Evaluation of a possible swelling-controlled drug release mechanism using dimensionless analysis. J. Control. Release 2010, 141, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Kranz, H.; Bodmeier, R.; Peppas, N.A. HPMC-matrices for controlled drug delivery: A new model combining diffusion, swelling, and dissolution mechanisms and predicting the release kinetics. Pharm. Res. 1999, 16, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.; Mchugh, A.J. A generalized diffusion–dissolution model for drug release from rigid polymer membrane matrices. J. Membr. Sci. 2011, 366, 104–115. [Google Scholar] [CrossRef]
- Cabrera, M.I.; Luna, J.A.; Grau, R.J.A. Modeling of dissolution-diffusion controlled drug release from planar polymeric systems with finite dissolution rate and arbitrary drug loading. J. Membr. Sci. 2006, 280, 693–704. [Google Scholar] [CrossRef]
- Choo, K.; Kim, I.; Jung, J.; Suh, Y.; Chung, S.; Lee, M.; Shim, C. Simultaneous determination of loxoprofen and its diastereomeric alcohol metabolites in human plasma and urine by a simple HPLC-UV detection method. J. Pharm. Biomed. Anal. 2001, 25, 639–650. [Google Scholar] [CrossRef]
- Badawy, S.I.; Williams, R.C.; Gilbert, D.L. Effect of different acids on solid-state stability of an ester prodrug of a IIb/IIIa glycoprotein receptor antagonist. Pharm. Dev. Technol. 1999, 4, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Siepe, S.; Lueckel, B.; Kramer, A.; Ries, A.; Gurny, R. Strategies for the design of hydrophilic matrix tablets with controlled microenvironmental pH. Int. J. Pharm. 2006, 316, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kazlauske, J.; Cafaro, M.M.; Caccavo, D.; Marucci, M.; Lamberti, G.; Barba, A.A.; Larsson, A. Determination of the release mechanism of Theophylline from pellets coated with Surelease(R)-A water dispersion of ethyl cellulose. Int. J. Pharm. 2017, 528, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration CDER. Guidance for Industry Q8 (R2) Pharmaceutical Development International Conference on Harmonisation of Techniccal Requirements for Registration of Pharmaceuticals for Human Use. 2009; pp. 1–19. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf (accessed on 6 May 2019).
- Charoo, N.A.; Shamsher, A.A.; Zidan, A.S.; Rahman, Z. Quality by design approach for formulation development: A case study of dispersible tablets. Int. J. Pharm. 2012, 423, 167–178. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Kawai, S.; Nishida, S.; Kato, M.; Furumaya, Y.; Okamoto, R.; Koshino, T.; Mizushima, Y. Comparison of cyclooxygenase-1 and -2 inhibitory activities of various nonsteroidal anti-inflammatory drugs using human platelets and synovial cells. Eur. J. Pharmacol. 1998, 347, 87–94. [Google Scholar] [CrossRef]
- Yamakawa, N.; Suemasu, S.; Kimoto, A.; Ishihara, T.; Yokomizo, K.; Okamoto, Y.; Otsuka, M. Low Direct Cytotoxicity of Loxoprofen on Gastric Mucosal Cells. Biol. Pharm. Bull. 2010, 33, 398. [Google Scholar] [CrossRef] [PubMed]
- Hatton, G.B.; Yadav, V.; Basit, A.W.; Merchant, H.A. Animal farm: Considerations in animal gastrointestinal physiology and relevance to drug delivery in humans. J. Pharm. Sci. 2015, 104, 2747–2776. [Google Scholar] [CrossRef] [PubMed]










| Independent Variables | Levels Used | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1 = citric acid concentration (%) | 1 | 2 | 3 |
| X2 = subcoating weight (%) | 6 | 8 | 10 |
| X3 = ADEC coating weight (%) | 10 | 13 | 16 |
| Responses | Constraints | ||
| Y1 = the drug release within 2 h | <30% | ||
| Y2 = the drug release within 6 h | 50–70% | ||
| Y3 = the drug release within 12 h | >90% | ||
| Formulations | Factors (%) | Responses (%) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | |
| 1 | 2.0 | 10.0 | 10.0 | 21.5 | 71.2 | 93.8 |
| 2 | 3.0 | 10.0 | 13.0 | 8.4 | 46.0 | 78.5 |
| 3 | 2.0 | 6.0 | 16.0 | 7.4 | 40.9 | 68.9 |
| 4 | 2.0 | 8.0 | 13.0 | 17.1 | 54.8 | 87.0 |
| 5 | 3.0 | 6.0 | 13.0 | 13.1 | 53.8 | 81.0 |
| 6 | 1.0 | 6.0 | 13.0 | 31.4 | 75.0 | 91.0 |
| 7 | 1.0 | 8.0 | 16.0 | 21.0 | 58.0 | 86.0 |
| 8 | 2.0 | 8.0 | 13.0 | 17.7 | 59.7 | 89.0 |
| 9 | 1.0 | 10.0 | 13.0 | 27.8 | 75.5 | 92.6 |
| 10 | 3.0 | 8.0 | 16.0 | 13.6 | 33.1 | 57.7 |
| 11 | 1.0 | 8.0 | 10.0 | 56.0 | 88.0 | 99.0 |
| 12 | 2.0 | 10.0 | 16.0 | 8.6 | 40.2 | 68.3 |
| 13 | 3.0 | 8.0 | 10.0 | 20.5 | 60.2 | 91.0 |
| 14 | 2.0 | 6.0 | 10.0 | 38.2 | 81.6 | 90.0 |
| 15 | 2.0 | 8.0 | 13.0 | 13.9 | 60.7 | 89.0 |
| Model Name | Equation |
|---|---|
| Zero-order model | Qt = k0t |
| First-order model | ln(Q0 − Qt) = −k1t + Q0 |
| Higuchi diffusion model | Qt = kHt1/2 |
| Ritger–Peppas model | lnQt = n lnt + k |
| Weibull distribution model | log[−ln(1 − Qt)] = b logt − loga |
| Hixson–Crowell model | (1 − Qt)1/3 = 1 − kt |
| Baker–Lonsdale model | 3/2 [ 1 − (1 − Qt)2/3] − Qt = kt |
| Term | Drug Release Within 2 h (Y1) | Drug Release Within 6 h (Y2) | Drug Release Within 12 h (Y3) | |||
|---|---|---|---|---|---|---|
| Cofficient | p-Value | Cofficient | p-Value | Cofficient | p-Value | |
| Constant | 16.23 | 0.000 | 58.40 | 0.000 | 88.33 | 0.000 * |
| X1 | −10.08 | 0.001 * | −12.91 | 0.001 * | −7.38 | 0.000 * |
| X2 | −2.98 | 0.007 * | −2.30 | 0.091 | 0.29 | 0.697 |
| X3 | −10.70 | 0.001 * | −16.11 | 0.001 * | −11.79 | 0.000 * |
| X1*X2 | −0.27 | 0.787 | −2.07 | 0.240 | −1.02 | 0.347 |
| X2*X3 | 4.47 | 0.006 * | 2.42 | 0.180 | −1.10 | 0.316 |
| X1*X3 | 7.03 | 0.000 * | 0.70 | 0.672 | −4.72 | 0.005 * |
| X1*X1 | 6.40 | 0.001 * | 2.78 | 0.148 | 0.13 | 0.902 |
| X2*X2 | −2.45 | 0.058 | 1.40 | 0.427 | 2.69 | 0.047 * |
| X3*X3 | 5.15 | 0.004 * | −1.33 | 0.451 | 5.39 | 0.003 * |
| Regression equation | Y1 = 16.23 − 10.08X1 − 2.98X2 − 10.7X3 − 4.47X2X3 + 7.03X1X3 + 6.4X12 + 5.15X32 | Y2 = 58.40 − 12.91X1 − 16.11X3 | Y3 = 88.33 − 7.38X1 − 11.79X3 − 4.72X1X3 − 2.69X22 − 5.39X32 | |||
| R-Squared | 0.9921 | 0.9865 | 0.9891 | |||
| Content | Model | Equation | r2 |
|---|---|---|---|
| loxoprofen | Zero-order model | Qt = 0.0833t + 0.0238 | 0.9288 |
| First-order model | ln(Q0 − Qt) − lnQ0 = −0.1787t + 0.0814 | 0.9794 | |
| Higuchi diffusion model | Qt = 0.3126t1/2 − 0.1496 | 0.9454 | |
| Ritger–Peppas model | lnQt = 0.7422 lnt + 2.7562 | 0.9597 | |
| Weibull distribution model | log[−ln(1 − Qt)] = 1.3840 logt − 1.0449 | 0.9944 | |
| Hixson–Crowell model | (1 − Qt)1/3 = −0.0514t + 1.0167 | 0.9874 |
| Pharmacokinetic Parameters | Cmax (µg/mL) | Tmax (h) | AUC0–12 (µg h/mL) | AUC0–∞ (µg h/mL) | Relative Bioavailability (%) |
|---|---|---|---|---|---|
| Optimal pellets (90 mg) | 2.60 ± 0.23 | 4.80 ± 0.57 | 12.77 ± 0.88 | 13.48 ± 0.94 | 87.16 ± 0.07 |
| Commercial tablets (60 mg) | 5.16 ± 0.60 | 0.60 ± 0.22 | 9.73 ± 0.61 | 10.31 ± 0.45 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, D.; Zhao, M.; Zhang, J.; Luan, L. Development and In Vitro-In Vivo Evaluation of a Novel Sustained-Release Loxoprofen Pellet with Double Coating Layer. Pharmaceutics 2019, 11, 260. https://doi.org/10.3390/pharmaceutics11060260
Wan D, Zhao M, Zhang J, Luan L. Development and In Vitro-In Vivo Evaluation of a Novel Sustained-Release Loxoprofen Pellet with Double Coating Layer. Pharmaceutics. 2019; 11(6):260. https://doi.org/10.3390/pharmaceutics11060260
Chicago/Turabian StyleWan, Dongwei, Min Zhao, Jingjing Zhang, and Libiao Luan. 2019. "Development and In Vitro-In Vivo Evaluation of a Novel Sustained-Release Loxoprofen Pellet with Double Coating Layer" Pharmaceutics 11, no. 6: 260. https://doi.org/10.3390/pharmaceutics11060260
APA StyleWan, D., Zhao, M., Zhang, J., & Luan, L. (2019). Development and In Vitro-In Vivo Evaluation of a Novel Sustained-Release Loxoprofen Pellet with Double Coating Layer. Pharmaceutics, 11(6), 260. https://doi.org/10.3390/pharmaceutics11060260